Systems kinomics for characterizing host responses to high-consequence pathogens at the NIH/NIAID Integrated Research Facility-Frederick
- PMID: 24585711
- PMCID: PMC6136422
- DOI: 10.1111/2049-632X.12163
Systems kinomics for characterizing host responses to high-consequence pathogens at the NIH/NIAID Integrated Research Facility-Frederick
Abstract
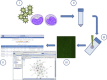
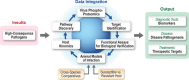
Currently, there is a paucity of information regarding the molecular pathogenesis for many high-consequence pathogens (HCPs) that pose threats to both national and international public health. In spite of this, investigations of the molecular pathogenesis for many HCPs have been limited to gross pathological changes in animal models or global analysis of gene expression. Further, questions remain regarding the ability of animal models of disease to recapitulate human molecular pathogenesis or act as predictors of therapeutic efficacy. Thus, it is likely that medical countermeasure development for HCPs will rely on identifying therapeutic targets that are uniquely modulated during HCP infection. It is also appreciated that many cellular processes can be regulated independently of changes in transcription or translation through phosphorylation events. Cellular kinases, individually or collectively (the kinome), play critical roles in regulating complex biology, underlie various malignancies, and represent high-priority drug targets. The growing interest in kinases in both basic and translational research has driven efforts to develop technologies that enable characterization of phosphorylation-mediated signal transduction. To this end, enhanced technical capabilities at the IRF-Frederick provide the unique capability for characterizing host responses to HCP insult during the course of infection and identify novel targets for therapeutic intervention.
Keywords: filovirus; high-consequence pathogens; kinase; kinome; orthopoxvirus; signaling pathways.
Published 2014. This article is a US Government work and is in the public domain in the USA.
Figures

References
-
- Allison M (2012) NCATS launches drug repurposing program. Nat Biotechnol 30: 571–572. - PubMed
-
- Arsenault R, Griebel P & Napper S (2011) Peptide arrays for kinome analysis: new opportunities and remaining challenges. Proteomics 11: 4595–4609. - PubMed
-
- Bhaumik S (2012) Twin Ebola outbreaks in Africa: Uganda and Democratic Republic of Congo affected. Natl Med J India 25: 317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

