Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins
- PMID: 24585742
- PMCID: PMC4061696
- DOI: 10.1161/CIRCGENETICS.113.000324
Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins
Abstract
Background: Hypertrophic cardiomyopathy (HCM) is a common genetic disorder caused mainly by mutations in sarcomeric proteins and is characterized by maladaptive myocardial hypertrophy, diastolic heart failure, increased myofilament Ca(2+) sensitivity, and high susceptibility to sudden death. We tested the following hypothesis: correction of the increased myofilament sensitivity can delay or prevent the development of the HCM phenotype.
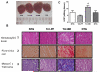
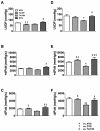
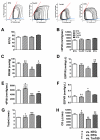
Methods and results: We used an HCM mouse model with an E180G mutation in α-tropomyosin (Tm180) that demonstrates increased myofilament Ca(2+) sensitivity, severe hypertrophy, and diastolic dysfunction. To test our hypothesis, we reduced myofilament Ca(2+) sensitivity in Tm180 mice by generating a double transgenic mouse line. We crossed Tm180 mice with mice expressing a pseudophosphorylated cardiac troponin I (S23D and S24D; TnI-PP). TnI-PP mice demonstrated a reduced myofilament Ca(2+) sensitivity compared with wild-type mice. The development of pathological hypertrophy did not occur in mice expressing both Tm180 and TnI-PP. Left ventricle performance was improved in double transgenic compared with their Tm180 littermates, which express wild-type cardiac troponin I. Hearts of double transgenic mice demonstrated no changes in expression of phospholamban and sarcoplasmic reticulum Ca(2+) ATPase, increased levels of phospholamban and troponin T phosphorylation, and reduced phosphorylation of TnI compared with Tm180 mice. Moreover, expression of TnI-PP in Tm180 hearts inhibited modifications in the activity of extracellular signal-regulated kinase and zinc finger-containing transcription factor GATA in Tm180 hearts.
Conclusions: Our data strongly indicate that reduction of myofilament sensitivity to Ca(2+) and associated correction of abnormal relaxation can delay or prevent development of HCM and should be considered as a therapeutic target for HCM.
Keywords: cardiac remodeling; cardiomyopathies; cardiomyopathy, hypertrophic; therapeutics.
Figures
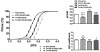




References
-
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2011;124:e783–831. - PubMed
-
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: An american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. - PubMed
-
- Hughes SE. The pathology of hypertrophic cardiomyopathy. Histopathology. 2004;44:412–427. - PubMed
-
- Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. - PubMed
-
- Bos JM, Towbin JA, Ackerman MJ. Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54:201–211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL-64035/HL/NHLBI NIH HHS/United States
- P01 HL-62426/HL/NHLBI NIH HHS/United States
- R01 HL-105924/HL/NHLBI NIH HHS/United States
- T32 HL-07692/HL/NHLBI NIH HHS/United States
- R01 HL-91056/HL/NHLBI NIH HHS/United States
- R01 HL081680/HL/NHLBI NIH HHS/United States
- P01 HL-077101/HL/NHLBI NIH HHS/United States
- P50 HL077101/HL/NHLBI NIH HHS/United States
- K99 HL091056/HL/NHLBI NIH HHS/United States
- R01 HL-81680/HL/NHLBI NIH HHS/United States
- R01 HL105924/HL/NHLBI NIH HHS/United States
- R01 HL064035/HL/NHLBI NIH HHS/United States
- P01 HL062426/HL/NHLBI NIH HHS/United States
- R01 HL079032/HL/NHLBI NIH HHS/United States
- P01 HL-49058/HL/NHLBI NIH HHS/United States
- R01 HL114940/HL/NHLBI NIH HHS/United States
- R00 HL091056/HL/NHLBI NIH HHS/United States
- T32 HL007692/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

