Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release
- PMID: 24586057
- PMCID: PMC4170115
- DOI: 10.1124/mol.114.091843
Differential effects of the Gβ5-RGS7 complex on muscarinic M3 receptor-induced Ca2+ influx and release
Abstract
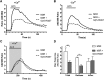
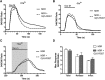
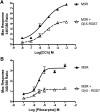
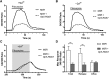
The G protein β subunit Gβ5 uniquely forms heterodimers with R7 family regulators of G protein signaling (RGS) proteins (RGS6, RGS7, RGS9, and RGS11) instead of Gγ. Although the Gβ5-RGS7 complex attenuates Ca(2+) signaling mediated by the muscarinic M3 receptor (M3R), the route of Ca(2+) entry (i.e., release from intracellular stores and/or influx across the plasma membrane) is unknown. Here, we show that, in addition to suppressing carbachol-stimulated Ca(2+) release, Gβ5-RGS7 enhanced Ca(2+) influx. This novel effect of Gβ5-RGS7 was blocked by nifedipine and 2-aminoethoxydiphenyl borate. Experiments with pertussis toxin, an RGS domain-deficient mutant of RGS7, and UBO-QIC {L-threonine,(3R)-N-acetyl-3-hydroxy-L-leucyl-(aR)-a-hydroxybenzenepropanoyl-2,3-idehydro-N-methylalanyl-L-alanyl-N-methyl-L-alanyl-(3R)-3-[[(2S,3R)-3-hydroxy-4- methyl-1-oxo-2-[(1-oxopropyl)amino]pentyl]oxy]-L-leucyl-N,O-dimethyl-,(7→1)-lactone (9CI)}, a novel inhibitor of Gq, showed that Gβ5-RGS7 modulated a Gq-mediated pathway. These studies indicate that Gβ5-RGS7, independent of RGS7 GTPase-accelerating protein activity, couples M3R to a nifedipine-sensitive Ca(2+) channel. We also compared the action of Gβ5-RGS7 on M3R-induced Ca(2+) influx and release elicited by different muscarinic agonists. Responses to Oxo-M [oxotremorine methiodide N,N,N,-trimethyl-4-(2-oxo-1-pyrrolidinyl)-2-butyn-1-ammonium iodide] were insensitive to Gβ5-RGS7. Pilocarpine responses consisted of a large release and modest influx components, of which the former was strongly inhibited whereas the latter was insensitive to Gβ5-RGS7. McN-A-343 [(4-hydroxy-2-butynyl)-1-trimethylammonium-3-chlorocarbanilate chloride] was the only compound whose total Ca(2+) response was enhanced by Gβ5-RGS7, attributed to, in part, by the relatively small Ca(2+) release this partial agonist stimulated. Together, these results show that distinct agonists not only have differential M3R functional selectivity, but also confer specific sensitivity to the Gβ5-RGS7 complex.
Figures

Similar articles
-
Helix 8 and the i3 loop of the muscarinic M3 receptor are crucial sites for its regulation by the Gβ5-RGS7 complex.Biochemistry. 2015 Feb 3;54(4):1077-88. doi: 10.1021/bi500980d. Epub 2015 Jan 20. Biochemistry. 2015. PMID: 25551629 Free PMC article.
-
Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion.FASEB J. 2017 Nov;31(11):4734-4744. doi: 10.1096/fj.201700197RR. Epub 2017 Jul 7. FASEB J. 2017. PMID: 28687610 Free PMC article.
-
The Gbeta5-RGS7 complex selectively inhibits muscarinic M3 receptor signaling via the interaction between the third intracellular loop of the receptor and the DEP domain of RGS7.Biochemistry. 2009 Mar 17;48(10):2282-9. doi: 10.1021/bi801989c. Biochemistry. 2009. PMID: 19182865 Free PMC article.
-
Structure, function, and localization of Gβ5-RGS complexes.Prog Mol Biol Transl Sci. 2009;86:157-203. doi: 10.1016/S1877-1173(09)86006-7. Epub 2009 Oct 7. Prog Mol Biol Transl Sci. 2009. PMID: 20374716 Free PMC article. Review.
-
R9AP and R7BP: traffic cops for the RGS7 family in phototransduction and neuronal GPCR signaling.Trends Pharmacol Sci. 2009 Jan;30(1):17-24. doi: 10.1016/j.tips.2008.10.002. Epub 2008 Nov 29. Trends Pharmacol Sci. 2009. PMID: 19042037 Free PMC article. Review.
Cited by
-
The orexin 1 receptor modulates kappa opioid receptor function via a JNK-dependent mechanism.Cell Signal. 2015 Jul;27(7):1449-56. doi: 10.1016/j.cellsig.2015.03.026. Epub 2015 Apr 6. Cell Signal. 2015. PMID: 25857454 Free PMC article.
-
On the selectivity of the Gαq inhibitor UBO-QIC: A comparison with the Gαi inhibitor pertussis toxin.Biochem Pharmacol. 2016 May 1;107:59-66. doi: 10.1016/j.bcp.2016.03.003. Epub 2016 Mar 5. Biochem Pharmacol. 2016. PMID: 26954502 Free PMC article.
-
Helix 8 and the i3 loop of the muscarinic M3 receptor are crucial sites for its regulation by the Gβ5-RGS7 complex.Biochemistry. 2015 Feb 3;54(4):1077-88. doi: 10.1021/bi500980d. Epub 2015 Jan 20. Biochemistry. 2015. PMID: 25551629 Free PMC article.
-
Regulator of G-protein signaling Gβ5-R7 is a crucial activator of muscarinic M3 receptor-stimulated insulin secretion.FASEB J. 2017 Nov;31(11):4734-4744. doi: 10.1096/fj.201700197RR. Epub 2017 Jul 7. FASEB J. 2017. PMID: 28687610 Free PMC article.
-
Oscillatory calcium release and sustained store-operated oscillatory calcium signaling prevents differentiation of human oligodendrocyte progenitor cells.Sci Rep. 2022 Apr 13;12(1):6160. doi: 10.1038/s41598-022-10095-1. Sci Rep. 2022. PMID: 35418597 Free PMC article.
References
-
- Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. (2006) RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal 18:579–591 - PubMed
-
- Baxter DF, Kirk M, Garcia AF, Raimondi A, Holmqvist MH, Flint KK, Bojanic D, Distefano PS, Curtis R, Xie Y. (2002) A novel membrane potential-sensitive fluorescent dye improves cell-based assays for ion channels. J Biomol Screen 7:79–85 - PubMed
-
- Blin N, Yun J, Wess J. (1995) Mapping of single amino acid residues required for selective activation of Gq/11 by the m3 muscarinic acetylcholine receptor. J Biol Chem 270:17741–17748 - PubMed
-
- Cabrera JL, de Freitas F, Satpaev DK, Slepak VZ. (1998) Identification of the Gbeta5-RGS7 protein complex in the retina. Biochem Biophys Res Commun 249:898–902 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

