Inflammatory monocytes orchestrate innate antifungal immunity in the lung
- PMID: 24586155
- PMCID: PMC3930594
- DOI: 10.1371/journal.ppat.1003940
Inflammatory monocytes orchestrate innate antifungal immunity in the lung
Abstract
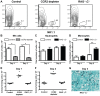
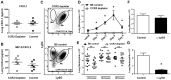
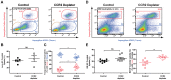
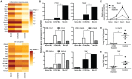
Aspergillus fumigatus is an environmental fungus that causes invasive aspergillosis (IA) in immunocompromised patients. Although -CC-chemokine receptor-2 (CCR2) and Ly6C-expressing inflammatory monocytes (CCR2⁺Mo) and their derivatives initiate adaptive pulmonary immune responses, their role in coordinating innate immune responses in the lung remain poorly defined. Using conditional and antibody-mediated cell ablation strategies, we found that CCR2⁺Mo and monocyte-derived dendritic cells (Mo-DCs) are essential for innate defense against inhaled conidia. By harnessing fluorescent Aspergillus reporter (FLARE) conidia that report fungal cell association and viability in vivo, we identify two mechanisms by which CCR2⁺Mo and Mo-DCs exert innate antifungal activity. First, CCR2⁺Mo and Mo-DCs condition the lung inflammatory milieu to augment neutrophil conidiacidal activity. Second, conidial uptake by CCR2⁺Mo temporally coincided with their differentiation into Mo-DCs, a process that resulted in direct conidial killing. Our findings illustrate both indirect and direct functions for CCR2⁺Mo and their derivatives in innate antifungal immunity in the lung.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

