Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth
- PMID: 24586159
- PMCID: PMC3930586
- DOI: 10.1371/journal.ppat.1003946
Identification of host-targeted small molecules that restrict intracellular Mycobacterium tuberculosis growth
Abstract
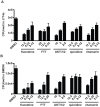
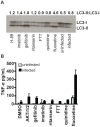
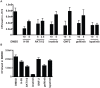
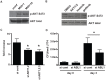
Mycobacterium tuberculosis remains a significant threat to global health. Macrophages are the host cell for M. tuberculosis infection, and although bacteria are able to replicate intracellularly under certain conditions, it is also clear that macrophages are capable of killing M. tuberculosis if appropriately activated. The outcome of infection is determined at least in part by the host-pathogen interaction within the macrophage; however, we lack a complete understanding of which host pathways are critical for bacterial survival and replication. To add to our understanding of the molecular processes involved in intracellular infection, we performed a chemical screen using a high-content microscopic assay to identify small molecules that restrict mycobacterial growth in macrophages by targeting host functions and pathways. The identified host-targeted inhibitors restrict bacterial growth exclusively in the context of macrophage infection and predominantly fall into five categories: G-protein coupled receptor modulators, ion channel inhibitors, membrane transport proteins, anti-inflammatories, and kinase modulators. We found that fluoxetine, a selective serotonin reuptake inhibitor, enhances secretion of pro-inflammatory cytokine TNF-α and induces autophagy in infected macrophages, and gefitinib, an inhibitor of the Epidermal Growth Factor Receptor (EGFR), also activates autophagy and restricts growth. We demonstrate that during infection signaling through EGFR activates a p38 MAPK signaling pathway that prevents macrophages from effectively responding to infection. Inhibition of this pathway using gefitinib during in vivo infection reduces growth of M. tuberculosis in the lungs of infected mice. Our results support the concept that screening for inhibitors using intracellular models results in the identification of tool compounds for probing pathways during in vivo infection and may also result in the identification of new anti-tuberculosis agents that work by modulating host pathways. Given the existing experience with some of our identified compounds for other therapeutic indications, further clinically-directed study of these compounds is merited.
Conflict of interest statement
Sarah Stanley received a Helen Hay Whitney postdoctoral fellowship from 2008–2011 that was sponsored by Novartis. This does not alter our adherence to PLOS Pathogens policies on sharing data and materials.
Figures

References
-
- Deretic V, Singh S, Master S, Harris J, Roberts E, et al. (2006) Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cellular Microbiology 8: 719–727. - PubMed
-
- Malik ZA, Iyer SS, Kusner DJ (2001) Mycobacterium tuberculosis Phagosomes Exhibit Altered Calmodulin-Dependent Signal Transduction: Contribution to Inhibition of Phagosome-Lysosome Fusion and Intracellular Survival in Human Macrophages. The Journal of Immunology 166: 3392–3401. - PubMed
-
- Jayachandran R, Sundaramurthy V, Combaluzier B, Mueller P, Korf H, et al. (2007) Survival of Mycobacteria in Macrophages Is Mediated by Coronin 1-Dependent Activation of Calcineurin. Cell 130: 37–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

