Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in Type-I interferon response
- PMID: 24586175
- PMCID: PMC3937324
- DOI: 10.1371/journal.ppat.1003981
Human genome-wide RNAi screen identifies an essential role for inositol pyrophosphates in Type-I interferon response
Erratum in
- PLoS Pathog. 2014 Mar;10(3):e1004095
- PLoS Pathog. 2014 Oct;10(10):e1004519. McCaffrey, Kathleen [added]
Abstract
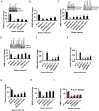
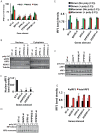
The pattern recognition receptor RIG-I is critical for Type-I interferon production. However, the global regulation of RIG-I signaling is only partially understood. Using a human genome-wide RNAi-screen, we identified 226 novel regulatory proteins of RIG-I mediated interferon-β production. Furthermore, the screen identified a metabolic pathway that synthesizes the inositol pyrophosphate 1-IP7 as a previously unrecognized positive regulator of interferon production. Detailed genetic and biochemical experiments demonstrated that the kinase activities of IPPK, PPIP5K1 and PPIP5K2 (which convert IP5 to1-IP7) were critical for both interferon induction, and the control of cellular infection by Sendai and influenza A viruses. Conversely, ectopically expressed inositol pyrophosphate-hydrolases DIPPs attenuated interferon transcription. Mechanistic experiments in intact cells revealed that the expression of IPPK, PPIP5K1 and PPIP5K2 was needed for the phosphorylation and activation of IRF3, a transcription factor for interferon. The addition of purified individual inositol pyrophosphates to a cell free reconstituted RIG-I signaling assay further identified 1-IP7 as an essential component required for IRF3 activation. The inositol pyrophosphate may act by β-phosphoryl transfer, since its action was not recapitulated by a synthetic phosphonoacetate analogue of 1-IP7. This study thus identified several novel regulators of RIG-I, and a new role for inositol pyrophosphates in augmenting innate immune responses to viral infection that may have therapeutic applications.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Fine-Tuning of the RIG-I-Like Receptor/Interferon Regulatory Factor 3-Dependent Antiviral Innate Immune Response by the Glycogen Synthase Kinase 3/β-Catenin Pathway.Mol Cell Biol. 2015 Sep 1;35(17):3029-43. doi: 10.1128/MCB.00344-15. Epub 2015 Jun 22. Mol Cell Biol. 2015. PMID: 26100021 Free PMC article.
-
RIG-I Signaling Is Essential for Influenza B Virus-Induced Rapid Interferon Gene Expression.J Virol. 2015 Dec;89(23):12014-25. doi: 10.1128/JVI.01576-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378160 Free PMC article.
-
TRIM11 negatively regulates IFNβ production and antiviral activity by targeting TBK1.PLoS One. 2013 May 13;8(5):e63255. doi: 10.1371/journal.pone.0063255. Print 2013. PLoS One. 2013. PMID: 23675467 Free PMC article.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
A new role for myeloid HO-1 in the innate to adaptive crosstalk and immune homeostasis.Adv Exp Med Biol. 2011;780:101-11. doi: 10.1007/978-1-4419-5632-3_9. Adv Exp Med Biol. 2011. PMID: 21842368 Review.
Cited by
-
Echinacea purpurea (L.) Moench treatment of monocytes promotes tonic interferon signaling, increased innate immunity gene expression and DNA repeat hypermethylated silencing of endogenous retroviral sequences.BMC Complement Med Ther. 2021 May 12;21(1):141. doi: 10.1186/s12906-021-03310-5. BMC Complement Med Ther. 2021. PMID: 33980308 Free PMC article.
-
Vip1 is a kinase and pyrophosphatase switch that regulates inositol diphosphate signaling.Proc Natl Acad Sci U S A. 2020 Apr 28;117(17):9356-9364. doi: 10.1073/pnas.1908875117. Epub 2020 Apr 17. Proc Natl Acad Sci U S A. 2020. PMID: 32303658 Free PMC article.
-
Synthetic inositol phosphate analogs reveal that PPIP5K2 has a surface-mounted substrate capture site that is a target for drug discovery.Chem Biol. 2014 May 22;21(5):689-99. doi: 10.1016/j.chembiol.2014.03.009. Epub 2014 Apr 24. Chem Biol. 2014. PMID: 24768307 Free PMC article.
-
Analyses of Inositol Phosphates and Phosphoinositides by Strong Anion Exchange (SAX)-HPLC.Methods Mol Biol. 2021;2295:365-378. doi: 10.1007/978-1-0716-1362-7_20. Methods Mol Biol. 2021. PMID: 34047987
-
Primate lentiviruses require Inositol hexakisphosphate (IP6) or inositol pentakisphosphate (IP5) for the production of viral particles.PLoS Pathog. 2020 Aug 10;16(8):e1008646. doi: 10.1371/journal.ppat.1008646. eCollection 2020 Aug. PLoS Pathog. 2020. PMID: 32776974 Free PMC article.
References
-
- Kumar H, Kawai T, Akira S (2009) Pathogen recognition in the innate immune response. Biochem J 420: 1–16. - PubMed
-
- Yoneyama M, Fujita T (2010) Recognition of viral nucleic acids in innate immunity. Rev Med Virol 20: 4–22. - PubMed
-
- Belgnaoui SM, Paz S, Hiscott J (2011) Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr Opin Immunol 23: 564–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

