Highly active antiretroviral therapies are effective against HIV-1 cell-to-cell transmission
- PMID: 24586176
- PMCID: PMC3937346
- DOI: 10.1371/journal.ppat.1003982
Highly active antiretroviral therapies are effective against HIV-1 cell-to-cell transmission
Abstract
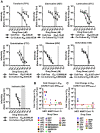
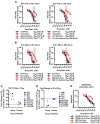
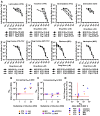
HIV-1 cell-to-cell transmission allows for 2-3 orders of magnitude more efficient viral spread than cell-free dissemination. The high local multiplicity of infection (MOI) observed at cell-cell contact sites may lower the efficacy of antiretroviral therapies (ART). Here we test the efficacy of commonly used antiretroviral inhibitors against cell-to-cell and cell-free HIV-1 transmission. We demonstrate that, while some nucleoside-analog reverse transcriptase inhibitors (NRTI) are less effective against HIV-1 cell-to-cell transmission, most non-nucleoside-analog reverse transcriptase inhibitors (NNRTI), entry inhibitors and protease inhibitors remain highly effective. Moreover, poor NRTIs become highly effective when applied in combinations explaining the effectiveness of ART in clinical settings. Investigating the underlying mechanism, we observe a strict correlation between the ability of individual drugs and combinations of drugs to interfere with HIV-1 cell-to-cell transmission, and their effectiveness against high viral MOIs. Our results suggest that the ability to suppress high viral MOI is a feature of effective ART regimens and this parameter should be considered when designing novel antiviral therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, et al. (1997) Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387: 188–191. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, et al. (1997) Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. The New England journal of medicine 337: 734–739. - PubMed
-
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, et al. (2006) The survival benefits of AIDS treatment in the United States. The Journal of infectious diseases 194: 11–19. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. (1997) A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. The New England journal of medicine 337: 725–733. - PubMed
-
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, et al. (2009) The challenge of finding a cure for HIV infection. Science 323: 1304–1307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

