Fate of clinical research studies after ethical approval--follow-up of study protocols until publication
- PMID: 24586265
- PMCID: PMC3929354
- DOI: 10.1371/journal.pone.0087184
Fate of clinical research studies after ethical approval--follow-up of study protocols until publication
Abstract
Background: Many clinical studies are ultimately not fully published in peer-reviewed journals. Underreporting of clinical research is wasteful and can result in biased estimates of treatment effect or harm, leading to recommendations that are inappropriate or even dangerous.
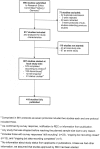
Methods: We assembled a cohort of clinical studies approved 2000-2002 by the Research Ethics Committee of the University of Freiburg, Germany. Published full articles were searched in electronic databases and investigators contacted. Data on study characteristics were extracted from protocols and corresponding publications. We characterized the cohort, quantified its publication outcome and compared protocols and publications for selected aspects.
Results: Of 917 approved studies, 807 were started and 110 were not, either locally or as a whole. Of the started studies, 576 (71%) were completed according to protocol, 128 (16%) discontinued and 42 (5%) are still ongoing; for 61 (8%) there was no information about their course. We identified 782 full publications corresponding to 419 of the 807 initiated studies; the publication proportion was 52% (95% CI: 0.48-0.55). Study design was not significantly associated with subsequent publication. Multicentre status, international collaboration, large sample size and commercial or non-commercial funding were positively associated with subsequent publication. Commercial funding was mentioned in 203 (48%) protocols and in 205 (49%) of the publications. In most published studies (339; 81%) this information corresponded between protocol and publication. Most studies were published in English (367; 88%); some in German (25; 6%) or both languages (27; 6%). The local investigators were listed as (co-)authors in the publications corresponding to 259 (62%) studies.
Conclusion: Half of the clinical research conducted at a large German university medical centre remains unpublished; future research is built on an incomplete database. Research resources are likely wasted as neither health care professionals nor patients nor policy makers can use the results when making decisions.
Conflict of interest statement
Figures
Similar articles
-
Clinical research projects at a German medical faculty: follow-up from ethical approval to publication and citation by others.J Med Ethics. 2008 Sep;34(9):e20. doi: 10.1136/jme.2008.024521. J Med Ethics. 2008. PMID: 18757621
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Extent of non-publication in cohorts of studies approved by research ethics committees or included in trial registries.PLoS One. 2014 Dec 23;9(12):e114023. doi: 10.1371/journal.pone.0114023. eCollection 2014. PLoS One. 2014. PMID: 25536072 Free PMC article.
-
[Ethics in articles published in medical journals].Rev Med Chil. 2007 Apr;135(4):529-33. Epub 2007 May 16. Rev Med Chil. 2007. PMID: 17554464 Review. Spanish.
-
Publication ethics.Paediatr Anaesth. 2009 Oct;19(10):1011-3. doi: 10.1111/j.1460-9592.2009.03086.x. Epub 2009 Jun 13. Paediatr Anaesth. 2009. PMID: 19619189 Review.
Cited by
-
Analysis of the discontinuation and nonpublication of neurooncological randomized clinical trials.Neurooncol Adv. 2024 Aug 1;6(1):vdae136. doi: 10.1093/noajnl/vdae136. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 39211519 Free PMC article.
-
Write-up and dissemination of undergraduate and postgraduate research at the University of Rwanda: a cross-sectional study.Pan Afr Med J. 2019 Apr 9;32:164. doi: 10.11604/pamj.2019.32.164.18409. eCollection 2019. Pan Afr Med J. 2019. PMID: 31303933 Free PMC article.
-
Reporting results of research involving human subjects: an ethical obligation.J Korean Med Sci. 2015 Jun;30(6):673-5. doi: 10.3346/jkms.2015.30.6.673. Epub 2015 May 13. J Korean Med Sci. 2015. PMID: 26028915 Free PMC article. Review.
-
Nonpublication Rates and Characteristics of Registered Randomized Clinical Trials in Digital Health: Cross-Sectional Analysis.J Med Internet Res. 2018 Dec 18;20(12):e11924. doi: 10.2196/11924. J Med Internet Res. 2018. PMID: 30485832 Free PMC article.
-
Publication rates of research projects of an internal funding program of a university medical center in Germany: A retrospective study (2004-2013).PLoS One. 2020 Nov 30;15(11):e0243092. doi: 10.1371/journal.pone.0243092. eCollection 2020. PLoS One. 2020. PMID: 33253269 Free PMC article.
References
-
- Rosenthal R (1979) The “file drawer problem” and tolerance for null results. Psychological Bulletin 86: 638–641.
-
- Dickersin K, Min YI, Meinert CL (1992) Factors influencing publication of research results. Follow-up of applications submitted to two institutional review boards. JAMA 267: 374–378. - PubMed
-
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR (1991) Publication bias in clinical research. Lancet 337: 867–872. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources