Generation of recombinant antibodies to rat GABAA receptor subunits by affinity selection on synthetic peptides
- PMID: 24586298
- PMCID: PMC3929611
- DOI: 10.1371/journal.pone.0087964
Generation of recombinant antibodies to rat GABAA receptor subunits by affinity selection on synthetic peptides
Abstract
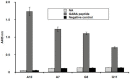
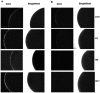
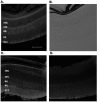
The abundance and physiological importance of GABAA receptors in the central nervous system make this neurotransmitter receptor an attractive target for localizing diagnostic and therapeutic biomolecules. GABAA receptors are expressed within the retina and mediate synaptic signaling at multiple stages of the visual process. To generate monoclonal affinity reagents that can specifically recognize GABAA receptor subunits, we screened two bacteriophage M13 libraries, which displayed human scFvs, by affinity selection with synthetic peptides predicted to correspond to extracellular regions of the rat α1 and β2 GABAA subunits. We isolated three anti-β2 and one anti-α1 subunit specific scFvs. Fluorescence polarization measurements revealed all four scFvs to have low micromolar affinities with their cognate peptide targets. The scFvs were capable of detecting fully folded GABAA receptors heterologously expressed by Xenopus laevis oocytes, while preserving ligand-gated channel activity. Moreover, A10, the anti-α1 subunit-specific scFv, was capable of detecting native GABAA receptors in the mouse retina, as observed by immunofluorescence staining. In order to improve their apparent affinity via avidity, we dimerized the A10 scFv by fusing it to the Fc portion of the IgG. The resulting scFv-Fc construct had a Kd of ∼26 nM, which corresponds to an approximately 135-fold improvement in binding, and a lower detection limit in dot blots, compared to the monomeric scFv. These results strongly support the use of peptides as targets for generating affinity reagents to membrane proteins and encourage investigation of molecular conjugates that use scFvs as anchoring components to localize reagents of interest at GABAA receptors of retina and other neural tissues, for studies of receptor activation and subunit structure.
Conflict of interest statement
Figures


References
-
- Zucker CL, Ehinger B (1998) Gamma-aminobutyric acidA receptors on a bistratified amacrine cell type in the rabbit retina. J Comp Neurol 393: 309–319. - PubMed
-
- Yang XL (2004) Characterization of receptors for glutamate and GABA in retinal neurons. Prog Neurobiol 73: 127–150. - PubMed
-
- Macdonald RL, Olsen RW (1994) GABAA receptor channels. Annu Rev Neurosci 17: 569–602. - PubMed
-
- Mohler H, Malherbe P, Draguhn A, Richards JG (1990) GABAA-receptors: structural requirements and sites of gene expression in mammalian brain. Neurochem Res 15: 199–207. - PubMed
-
- Seeburg PH, Wisden W, Verdoorn TA, Pritchett DB, Werner P, et al. (1990) The GABAA receptor family: molecular and functional diversity. Cold Spring Harb Symp Quant Biol 55: 29–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

