A voltage dependent non-inactivating Na+ channel activated during apoptosis in Xenopus oocytes
- PMID: 24586320
- PMCID: PMC3938416
- DOI: 10.1371/journal.pone.0088381
A voltage dependent non-inactivating Na+ channel activated during apoptosis in Xenopus oocytes
Abstract
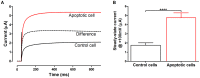
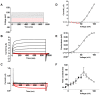
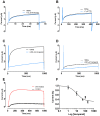
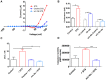
Ion channels in the plasma membrane are important for the apoptotic process. Different types of voltage-gated ion channels are up-regulated early in the apoptotic process and block of these channels prevents or delays apoptosis. In the present investigation we examined whether ion channels are up-regulated in oocytes from the frog Xenopus laevis during apoptosis. The two-electrode voltage-clamp technique was used to record endogenous ion currents in the oocytes. During staurosporine-induced apoptosis a voltage-dependent Na(+) current increased three-fold. This current was activated at voltages more positive than 0 mV (midpoint of the open-probability curve was +55 mV) and showed almost no sign of inactivation during a 1-s pulse. The current was resistant to the Na(+)-channel blockers tetrodotoxin (1 µM) and amiloride (10 µM), while the Ca(2+)-channel blocker verapamil (50 µM) in the bath solution completely blocked the current. The intracellular Na(+) concentration increased in staurosporine-treated oocytes, but could be prevented by replacing extracellular Na(+) with either K(+) or Choline(+). Prevention of this influx of Na(+) also prevented the STS-induced up-regulation of the caspase-3 activity, suggesting that the intracellular Na(+) increase is required to induce apoptosis. Taken together, we have found that a voltage dependent Na(+) channel is up-regulated during apoptosis and that influx of Na(+) is a crucial step in the apoptotic process in Xenopus oocytes.
Conflict of interest statement
Figures




References
-
- Hughes FM, Bortner CD, Purdy GD, Cidlowski JA (1997) Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem 272: 30567–30576. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

