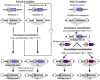
The kick-in system: a novel rapid knock-in strategy
- PMID: 24586341
- PMCID: PMC3929540
- DOI: 10.1371/journal.pone.0088549
The kick-in system: a novel rapid knock-in strategy
Abstract
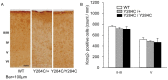
Knock-in mouse models have contributed tremendously to our understanding of human disorders. However, generation of knock-in animals requires a significant investment of time and effort. We addressed this problem by developing a novel knock-in system that circumvents several traditional challenges by establishing stem cells with acceptor elements enveloping a particular genomic target. Once established, these acceptor embryonic stem (ES) cells are efficient at directionally incorporating mutated target DNA using modified Cre/lox technology. This is advantageous, because knock-ins are not restricted to one a priori selected variation. Rather, it is possible to generate several mutant animal lines harboring desired alterations in the targeted area. Acceptor ES cell generation is the rate-limiting step, lasting approximately 2 months. Subsequent manipulations toward animal production require an additional 8 weeks, but this delimits the full period from conception of the genetic alteration to its animal incorporation. We call this system a "kick-in" to emphasize its unique characteristics of speed and convenience. To demonstrate the functionality of the kick-in methodology, we generated two mouse lines with separate mutant versions of the voltage-dependent potassium channel Kv7.2 (Kcnq2): p.Tyr284Cys (Y284C) and p.Ala306Thr (A306T); both variations have been associated with benign familial neonatal epilepsy. Adult mice homozygous for Y284C, heretofore unexamined in animals, presented with spontaneous seizures, whereas A306T homozygotes died early. Heterozygous mice of both lines showed increased sensitivity to pentylenetetrazole, possibly due to a reduction in M-current in CA1 hippocampal pyramidal neurons. Our observations for the A306T animals match those obtained with traditional knock-in technology, demonstrating that the kick-in system can readily generate mice bearing various mutations, making it a suitable feeder technology toward streamlined phenotyping.
Conflict of interest statement
Figures
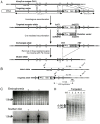



 ), and homozygous (•) Y284C mice at 4 weeks (B) and 10 weeks (C). Whole-cell perforated patched CA1 neurons were stepped to the indicated potential and back to −20 mV to determine tail current amplitudes. Significant I
M reductions were seen in Y284C homozygotes at 4 and 10 weeks, and in heterozygotes at 10 weeks at −60 mV (P<0.05, Tukey-Kramer test).
), and homozygous (•) Y284C mice at 4 weeks (B) and 10 weeks (C). Whole-cell perforated patched CA1 neurons were stepped to the indicated potential and back to −20 mV to determine tail current amplitudes. Significant I
M reductions were seen in Y284C homozygotes at 4 and 10 weeks, and in heterozygotes at 10 weeks at −60 mV (P<0.05, Tukey-Kramer test).Similar articles
-
Retigabine, a Kv7.2/Kv7.3-Channel Opener, Attenuates Drug-Induced Seizures in Knock-In Mice Harboring Kcnq2 Mutations.PLoS One. 2016 Feb 24;11(2):e0150095. doi: 10.1371/journal.pone.0150095. eCollection 2016. PLoS One. 2016. PMID: 26910900 Free PMC article.
-
Mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions show seizures and neuronal plasticity without synaptic reorganization.J Physiol. 2008 Jul 15;586(14):3405-23. doi: 10.1113/jphysiol.2008.154971. Epub 2008 May 15. J Physiol. 2008. PMID: 18483067 Free PMC article.
-
Electroconvulsive seizure thresholds and kindling acquisition rates are altered in mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions.Epilepsia. 2009 Jul;50(7):1752-9. doi: 10.1111/j.1528-1167.2009.02100.x. Epub 2009 Apr 27. Epilepsia. 2009. PMID: 19453707
-
Potassium channel genes and benign familial neonatal epilepsy.Prog Brain Res. 2014;213:17-53. doi: 10.1016/B978-0-444-63326-2.00002-8. Prog Brain Res. 2014. PMID: 25194482 Review.
-
Sodium and potassium channel dysfunctions in rare and common idiopathic epilepsy syndromes.Brain Dev. 2009 Aug;31(7):515-20. doi: 10.1016/j.braindev.2009.04.012. Epub 2009 May 22. Brain Dev. 2009. PMID: 19464834 Review.
Cited by
-
Infantile spasms and encephalopathy without preceding neonatal seizures caused by KCNQ2 R198Q, a gain-of-function variant.Epilepsia. 2017 Jan;58(1):e10-e15. doi: 10.1111/epi.13601. Epub 2016 Nov 9. Epilepsia. 2017. PMID: 27861786 Free PMC article.
-
The Role of Kv7.2 in Neurodevelopment: Insights and Gaps in Our Understanding.Front Physiol. 2020 Oct 28;11:570588. doi: 10.3389/fphys.2020.570588. eCollection 2020. Front Physiol. 2020. PMID: 33192566 Free PMC article. Review.
-
Mouse models of Kcnq2 dysfunction.Epilepsia. 2022 Nov;63(11):2813-2826. doi: 10.1111/epi.17405. Epub 2022 Sep 27. Epilepsia. 2022. PMID: 36047730 Free PMC article. Review.
-
Retigabine, a Kv7.2/Kv7.3-Channel Opener, Attenuates Drug-Induced Seizures in Knock-In Mice Harboring Kcnq2 Mutations.PLoS One. 2016 Feb 24;11(2):e0150095. doi: 10.1371/journal.pone.0150095. eCollection 2016. PLoS One. 2016. PMID: 26910900 Free PMC article.
-
A knock-in mouse model for KCNQ2-related epileptic encephalopathy displays spontaneous generalized seizures and cognitive impairment.Epilepsia. 2020 May;61(5):868-878. doi: 10.1111/epi.16494. Epub 2020 Apr 2. Epilepsia. 2020. PMID: 32239694 Free PMC article.
References
-
- Hirose S, Mitsudome A, Okada M, Kaneko S (2005) Epilepsy Genetic Study Group J (2005) Genetics of idiopathic epilepsies. Epilepsia 46 Suppl 138–43. - PubMed
-
- Kaneko S, Okada M, Iwasa H, Yamakawa K, Hirose S (2002) Genetics of epilepsy: current status and perspectives. Neurosci Res 44: 11–30. - PubMed
-
- Ronen GM, Rosales TO, Connolly M, Anderson VE, Leppert M (1993) Seizure characteristics in chromosome 20 benign familial neonatal convulsions. Neurology 43: 1355–1360. - PubMed
-
- Vigevano F (2005) Benign familial infantile seizures. Brain Dev 27: 172–177. - PubMed
-
- Dedek K, Fusco L, Teloy N, Steinlein OK (2003) Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy Res 54: 21–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

