C. elegans CEP-1/p53 and BEC-1 are involved in DNA repair
- PMID: 24586407
- PMCID: PMC3930633
- DOI: 10.1371/journal.pone.0088828
C. elegans CEP-1/p53 and BEC-1 are involved in DNA repair
Abstract
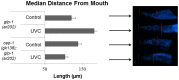
p53 is a transcription factor that regulates the response to cellular stress. Mammalian p53 functions as a tumor suppressor. The C. elegans p53, cep-1, regulates DNA-damage induced germline cell death by activating the transcription of egl-1 and ced-13. We used the C. elegans model to investigate how, in the whole animal, different forms of DNA damage can induce p53-dependent versus p53-independent cell death and DNA repair. DNA damage was induced by ultraviolet type C (UVC) radiation, or 10-decarbamoyl mitomycin C (DMC, an agent known to induce mammalian p53-independent cell death). Wild-type or cep-1 loss-of-function mutant animals were assayed for germline cell death and DNA lesions. Wild-type animals displayed greater removal of UVC-lesions over time, whereas cep-1 mutant animals displayed increased UVC-lesion retention. The cep-1 mutation increased UVC-lesion retention directly correlated with a reduction of progeny viability. Consistent with DMC inducing p53-independent cell death in mammalian cells DMC induced a C. elegans p53-independent germline cell death pathway. To examine the influence of wild-type CEP-1 and DNA damage on C. elegans tumors we used glp-1(ar202gf)/Notch germline tumor mutants. UVC treatment of glp-1 mutant animals activated the CEP-1 target gene egl-1 and reduced tumor size. In cep-1(gk138);glp-1(ar202gf) animals, UVC treatment resulted in increased susceptibility to lesions and larger tumorous germlines. Interestingly, the partial knockdown of bec-1 in adults resulted in a CEP-1-dependent increase in germline cell death and an increase in DNA damage. These results strongly support cross-talk between BEC-1 and CEP-1 to protect the C. elegans genome.
Conflict of interest statement
Figures
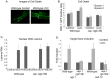
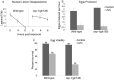

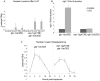
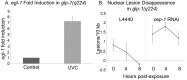

Similar articles
-
Mutant C. elegans p53 Together with Gain-of-Function GLP-1/Notch Decreases UVC-Damage-Induced Germline Cell Death but Increases PARP Inhibitor-Induced Germline Cell Death.Cancers (Basel). 2022 Oct 8;14(19):4929. doi: 10.3390/cancers14194929. Cancers (Basel). 2022. PMID: 36230851 Free PMC article.
-
Transcriptional profiling in C. elegans suggests DNA damage dependent apoptosis as an ancient function of the p53 family.BMC Genomics. 2008 Jul 15;9:334. doi: 10.1186/1471-2164-9-334. BMC Genomics. 2008. PMID: 18627611 Free PMC article.
-
Stress-Induced Sleep After Exposure to Ultraviolet Light Is Promoted by p53 in Caenorhabditis elegans.Genetics. 2017 Oct;207(2):571-582. doi: 10.1534/genetics.117.300070. Epub 2017 Jul 28. Genetics. 2017. PMID: 28754659 Free PMC article.
-
Germline survival and apoptosis.WormBook. 2008 Sep 4:1-20. doi: 10.1895/wormbook.1.145.1. WormBook. 2008. PMID: 18781708 Free PMC article. Review.
-
DNA repair.WormBook. 2006 Jan 13:1-12. doi: 10.1895/wormbook.1.54.1. WormBook. 2006. PMID: 18050489 Free PMC article. Review.
Cited by
-
Non-linear impact of glutathione depletion on C. elegans life span and stress resistance.Redox Biol. 2017 Apr;11:502-515. doi: 10.1016/j.redox.2016.12.003. Epub 2016 Dec 6. Redox Biol. 2017. PMID: 28086197 Free PMC article.
-
Sulfonate-Modified Polystyrene Nanoparticle at Precited Environmental Concentrations Induces Transgenerational Toxicity Associated with Increase in Germline Notch Signal of Caenorhabditis elegans.Toxics. 2023 Jun 6;11(6):511. doi: 10.3390/toxics11060511. Toxics. 2023. PMID: 37368611 Free PMC article.
-
The C-terminus of Gain-of-Function Mutant p53 R273H Is Required for Association with PARP1 and Poly-ADP-Ribose.Mol Cancer Res. 2022 Dec 2;20(12):1799-1810. doi: 10.1158/1541-7786.MCR-22-0133. Mol Cancer Res. 2022. PMID: 36074101 Free PMC article.
-
A CANCER PERSISTENT DNA REPAIR CIRCUIT DRIVEN BY MDM2, MDM4 (MDMX), AND MUTANT P53 FOR RECRUITMENT OF MDC1 AND 53BP1 TO CHROMATIN.bioRxiv [Preprint]. 2024 Jan 23:2024.01.20.576487. doi: 10.1101/2024.01.20.576487. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jul 8;53(13):gkaf627. doi: 10.1093/nar/gkaf627. PMID: 38328189 Free PMC article. Updated. Preprint.
-
Role of the tumour protein P53 gene in human cervical squamous carcinoma cells: Discussing haematopoietic cell-specific protein 1-associated protein X-1-induced survival, migration and proliferation.Oncol Lett. 2018 Aug;16(2):2629-2637. doi: 10.3892/ol.2018.8886. Epub 2018 Jun 4. Oncol Lett. 2018. PMID: 30013658 Free PMC article.
References
-
- Riley T, Sontag E, Chen P, Levine A (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9: 402–412. - PubMed
-
- Schumacher B, Hofmann K, Boulton S, Gartner A (2001) The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr Biol 11: 1722–1727. - PubMed
-
- Derry WB, Putzke AP, Rothman JH (2001) Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294: 591–595. - PubMed
-
- Lu WJ, Abrams JM (2006) Lessons from p53 in non-mammalian models. Cell Death Differ 13: 909–912. - PubMed
-
- Derry WB, Bierings R, van Iersel M, Satkunendran T, Reinke V, et al. (2007) Regulation of developmental rate and germ cell proliferation in Caenorhabditis elegans by the p53 gene network. Cell Death Differ 14: 662–670. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

