Bovine papillomavirus type 2 (BPV-2) E5 oncoprotein binds to the subunit D of the V₁-ATPase proton pump in naturally occurring urothelial tumors of the urinary bladder of cattle
- PMID: 24586417
- PMCID: PMC3933332
- DOI: 10.1371/journal.pone.0088860
Bovine papillomavirus type 2 (BPV-2) E5 oncoprotein binds to the subunit D of the V₁-ATPase proton pump in naturally occurring urothelial tumors of the urinary bladder of cattle
Abstract
Background: Active infection by bovine papillomavirus type 2 (BPV-2) was documented for fifteen urinary bladder tumors in cattle. Two were diagnosed as papillary urothelial neoplasm of low malignant potential (PUNLMP), nine as papillary and four as invasive urothelial cancers.
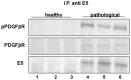
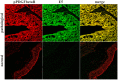
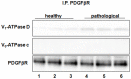
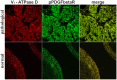
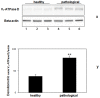
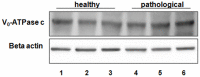
Methods and findings: In all cancer samples, PCR analysis revealed a BPV-2-specific 503 bp DNA fragment. E5 protein, the major oncoprotein of the virus, was shown both by immunoprecipitation and immunohistochemical analysis. E5 was found to bind to the activated (phosphorylated) form of the platelet derived growth factor β receptor. PDGFβR immunoprecipitation from bladder tumor samples and from normal bladder tissue used as control revealed a protein band which was present in the pull-down from bladder cancer samples only. The protein was identified with mass spectrometry as "V₁-ATPase subunit D", a component of the central stalk of the V₁-ATPase vacuolar pump. The subunit D was confirmed in this complex by coimmunoprecipitation investigations and it was found to colocalize with the receptor. The subunit D was also shown to be overexpressed by Western blot, RT-PCR and immunofluorescence analyses. Immunoprecipitation and immunofluorescence also revealed that E5 oncoprotein was bound to the subunit D.
Conclusion: For the first time, a tri-component complex composed of E5/PDGFβR/subunit D has been documented in vivo. Previous in vitro studies have shown that the BPV-2 E5 oncoprotein binds to the proteolipid c ring of the V₀-ATPase sector. We suggest that the E5/PDGFβR/subunit D complex may perturb proteostasis, organelle and cytosol homeostasis, which can result in altered protein degradation and in autophagic responses.
Conflict of interest statement
Figures










Similar articles
-
Bovine papillomavirus E5 oncoprotein binds to the activated form of the platelet-derived growth factor beta receptor in naturally occurring bovine urinary bladder tumours.Oncogene. 2006 Feb 23;25(8):1251-60. doi: 10.1038/sj.onc.1209152. Oncogene. 2006. PMID: 16205631
-
Mitophagy mediated by BNIP3 and BNIP3L/NIX in urothelial cells of the urinary bladder of cattle harbouring bovine papillomavirus infection.Vet Microbiol. 2019 Sep;236:108396. doi: 10.1016/j.vetmic.2019.108396. Epub 2019 Aug 22. Vet Microbiol. 2019. PMID: 31500722
-
Bovine Papillomavirus Type 13 Expression in the Urothelial Bladder Tumours of Cattle.Transbound Emerg Dis. 2016 Dec;63(6):628-634. doi: 10.1111/tbed.12322. Epub 2015 Jan 19. Transbound Emerg Dis. 2016. PMID: 25597262
-
Role of BAG3 in bovine Deltapapillomavirus-mediated autophagy.J Cell Biochem. 2022 Jan;123(1):59-64. doi: 10.1002/jcb.30193. Epub 2021 Dec 10. J Cell Biochem. 2022. PMID: 34889472 Review.
-
The bovine papillomavirus E5 protein and the PDGF beta receptor: it takes two to tango.Virology. 2009 Feb 20;384(2):345-51. doi: 10.1016/j.virol.2008.09.033. Epub 2008 Nov 6. Virology. 2009. PMID: 18990418 Free PMC article. Review.
Cited by
-
Molecular findings and virological assessment of bladder papillomavirus infection in cattle.Vet Q. 2024 Dec;44(1):1-7. doi: 10.1080/01652176.2024.2387072. Epub 2024 Aug 4. Vet Q. 2024. PMID: 39097798 Free PMC article.
-
Molecular and Phylogenetic Analysis of Bovine Papillomavirus Type 1: First Report in Iraqi Cattle.Adv Virol. 2016;2016:2143024. doi: 10.1155/2016/2143024. Epub 2016 Jun 20. Adv Virol. 2016. PMID: 27413374 Free PMC article.
-
Digital droplet PCR-based detection and quantification of ovine papillomavirus DNA from the vaginal virobiota of healthy mares.Sci Rep. 2025 Mar 22;15(1):9951. doi: 10.1038/s41598-025-94279-5. Sci Rep. 2025. PMID: 40121289 Free PMC article.
-
Bovine delta papillomavirus E5 oncoprotein negatively regulates the cGAS-STING signaling pathway in cattle in a spontaneous model of viral disease.Front Immunol. 2022 Oct 12;13:937736. doi: 10.3389/fimmu.2022.937736. eCollection 2022. Front Immunol. 2022. PMID: 36311756 Free PMC article.
-
Pteridium spp. and Bovine Papillomavirus: Partners in Cancer.Front Vet Sci. 2021 Nov 2;8:758720. doi: 10.3389/fvets.2021.758720. eCollection 2021. Front Vet Sci. 2021. PMID: 34796228 Free PMC article. Review.
References
-
- Meuten DJ (2002) Tumors of the urinary system. In Meuten, D.J. (Ed) Tumors in Domestic Animals, Blackwell Publishing Company, Iowa State Press, Ames, 524–546.
-
- Özkul IA, Aydin Y (1996) Tumours of the urinary bladder in cattle and water buffalo in the Black Sea region of Turkey. Br Vet J 152: 473–475. - PubMed
-
- Pamukcu AM, Price JM, Bryan GT (1976) Naturally occurring and bracken fern-induced bovine urinary bladder tumors. Clinical and morphological characteristics. Vet Pathol 13: 110–122. - PubMed
-
- Roperto S, Borzacchiello G, Brun R, Leonardi L, Maiolino P, et al. (2010a) A review of bovine urothelial tumours and tumour-like lesions of the urinary bladder. J Comp Pathol 142: 95–108. - PubMed
-
- Evans IA, Mason J (1965) Carcinogenic activity of bracken. Nature 208: 913–914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

