Improvement of chloride transport defect by gonadotropin-releasing hormone (GnRH) in cystic fibrosis epithelial cells
- PMID: 24586461
- PMCID: PMC3929654
- DOI: 10.1371/journal.pone.0088964
Improvement of chloride transport defect by gonadotropin-releasing hormone (GnRH) in cystic fibrosis epithelial cells
Abstract
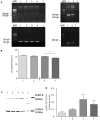
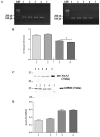
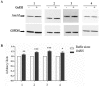
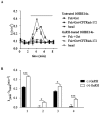
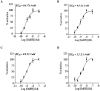
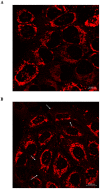
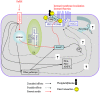
Cystic fibrosis (CF), the most common autosomal recessive disease in Caucasians, is due to mutations in the CFTR gene. F508del, the most frequent mutation in patients, impairs CFTR protein folding and biosynthesis. The F508del-CFTR protein is retained in the endoplasmic reticulum (ER) and its traffic to the plasma membrane is altered. Nevertheless, if it reaches the cell surface, it exhibits a Cl(-) channel function despite a short half-life. Pharmacological treatments may target the F508del-CFTR defect directly by binding to the mutant protein or indirectly by altering cellular proteostasis, and promote its plasma membrane targeting and stability. We previously showed that annexine A5 (AnxA5) directly binds to F508del-CFTR and, when overexpressed, promotes its membrane stability, leading to the restoration of some Cl(-) channel function in cells. Because Gonadotropin-Releasing Hormone (GnRH) increases AnxA5 expression in some cells, we tested it in CF cells. We showed that human epithelial cells express GnRH-receptors (GnRH-R) and that GnRH induces an AnxA5 overexpression and an increased Cl(-) channel function in F508del-CFTR cells, due to an increased stability of the protein in the membranes. Beside the numerous physiological implications of the GnRH-R expression in epithelial cells, we propose that a topical use of GnRH is a potential treatment in CF.
Conflict of interest statement
Figures




Similar articles
-
Buserelin alleviates chloride transport defect in human cystic fibrosis nasal epithelial cells.PLoS One. 2017 Nov 16;12(11):e0187774. doi: 10.1371/journal.pone.0187774. eCollection 2017. PLoS One. 2017. PMID: 29145426 Free PMC article.
-
F508del-CFTR increases intracellular Ca(2+) signaling that causes enhanced calcium-dependent Cl(-) conductance in cystic fibrosis.Biochim Biophys Acta. 2011 Nov;1812(11):1385-92. doi: 10.1016/j.bbadis.2011.08.008. Epub 2011 Aug 30. Biochim Biophys Acta. 2011. PMID: 21907281
-
The anion transporter SLC26A9 localizes to tight junctions and is degraded by the proteasome when co-expressed with F508del-CFTR.J Biol Chem. 2019 Nov 29;294(48):18269-18284. doi: 10.1074/jbc.RA119.010192. Epub 2019 Oct 23. J Biol Chem. 2019. PMID: 31645438 Free PMC article.
-
Targeting F508del-CFTR to develop rational new therapies for cystic fibrosis.Acta Pharmacol Sin. 2011 Jun;32(6):693-701. doi: 10.1038/aps.2011.71. Acta Pharmacol Sin. 2011. PMID: 21642944 Free PMC article. Review.
-
CFTR: folding, misfolding and correcting the ΔF508 conformational defect.Trends Mol Med. 2012 Feb;18(2):81-91. doi: 10.1016/j.molmed.2011.10.003. Epub 2011 Dec 3. Trends Mol Med. 2012. PMID: 22138491 Free PMC article. Review.
Cited by
-
Buserelin alleviates chloride transport defect in human cystic fibrosis nasal epithelial cells.PLoS One. 2017 Nov 16;12(11):e0187774. doi: 10.1371/journal.pone.0187774. eCollection 2017. PLoS One. 2017. PMID: 29145426 Free PMC article.
-
Gonadotropin-releasing hormone stimulates biliary proliferation by paracrine/autocrine mechanisms.Am J Pathol. 2015 Apr;185(4):1061-72. doi: 10.1016/j.ajpath.2014.12.004. Am J Pathol. 2015. PMID: 25794706 Free PMC article.
-
A novel role for CFTR interaction with LH and FGF in azoospermia and epididymal maldevelopment caused by cryptorchidism.Basic Clin Androl. 2022 Jun 21;32(1):10. doi: 10.1186/s12610-022-00160-0. Basic Clin Androl. 2022. PMID: 35725394 Free PMC article. Review.
-
Function and regulation of TRPM7, as well as intracellular magnesium content, are altered in cells expressing ΔF508-CFTR and G551D-CFTR.Cell Mol Life Sci. 2016 Sep;73(17):3351-73. doi: 10.1007/s00018-016-2149-6. Epub 2016 Feb 13. Cell Mol Life Sci. 2016. PMID: 26874684 Free PMC article.
References
-
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, et al. (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245: 1066–1073. - PubMed
-
- Bear CE, Li CH, Kartner N, Bridges RJ, Jensen TJ, et al. (1992) Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 68: 809–818. - PubMed
-
- Amaral MD (2004) Cftr and chaperones: Processing and degradation. J MolNeurosci 23: 41–48. - PubMed
-
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, et al. (1990) Defective intracellular transport and processing of cftr is the molecular basis of most cystic fibrosis. Cell 63: 827–834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

