Population structure and evolution of Rhinoviruses
- PMID: 24586469
- PMCID: PMC3929619
- DOI: 10.1371/journal.pone.0088981
Population structure and evolution of Rhinoviruses
Abstract
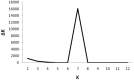
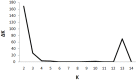
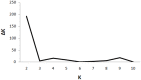
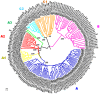
Rhinoviruses, formerly known as Human rhinoviruses, are the most common cause of air-borne upper respiratory tract infections in humans. Rhinoviruses belong to the family Picornaviridae and are divided into three species namely, Rhinovirus A, -B and -C, which are antigenically diverse. Genetic recombination is found to be one of the important causes for diversification of Rhinovirus species. Although emerging lineages within Rhinoviruses have been reported, their population structure has not been studied yet. The availability of complete genome sequences facilitates study of population structure, genetic diversity and underlying evolutionary forces, such as mutation, recombination and selection pressure. Analysis of complete genomes of Rhinoviruses using a model-based population genetics approach provided a strong evidence for existence of seven genetically distinct subpopulations. As a result of diversification, Rhinovirus A and -C populations are divided into four and two subpopulations, respectively. Genetically, the Rhinovirus B population was found to be homogeneous. Intra-species recombination was observed to be prominent in Rhinovirus A and -C species. Significant evidence of episodic positive selection was obtained for several sites within coding sequences of structural and non-structural proteins. This corroborates well with known phenotypic properties such as antigenicity of structural proteins. Episodic positive selection appears to be responsible for emergence of new lineages especially in Rhinovirus A. In summary, the Rhinovirus population is an ensemble of seven distinct lineages. In case of Rhinovirus A, intra-species recombination and episodic positive selection contribute to its further diversification. In case of Rhinovirus C, intra- and inter-species recombinations are responsible for observed diversity. Population genetics approach was further useful to analyze phylogenetic tree topologies pertaining to recombinant strains, especially when trees are derived using complete genomes. Understanding of population structure serves as a foundation for designing new vaccines and drugs as well as to explain emergence of drug resistance amongst subpopulations.
Conflict of interest statement
Figures

Similar articles
-
Recombination in the evolution of human rhinovirus genomes.Arch Virol. 2013 Jul;158(7):1497-515. doi: 10.1007/s00705-013-1634-6. Epub 2013 Feb 27. Arch Virol. 2013. PMID: 23443931
-
Phylogenetic relationships and molecular adaptation dynamics of human rhinoviruses.Mol Biol Evol. 2009 May;26(5):969-81. doi: 10.1093/molbev/msp009. Epub 2009 Jan 30. Mol Biol Evol. 2009. PMID: 19182223
-
Genome-wide diversity and selective pressure in the human rhinovirus.Virol J. 2007 May 3;4:40. doi: 10.1186/1743-422X-4-40. Virol J. 2007. PMID: 17477878 Free PMC article.
-
Proposals for the classification of human rhinovirus species C into genotypically assigned types.J Gen Virol. 2010 Oct;91(Pt 10):2409-19. doi: 10.1099/vir.0.023994-0. Epub 2010 Jul 7. J Gen Virol. 2010. PMID: 20610666 Review.
-
Rhinoviruses and Respiratory Enteroviruses: Not as Simple as ABC.Viruses. 2016 Jan 11;8(1):16. doi: 10.3390/v8010016. Viruses. 2016. PMID: 26761027 Free PMC article. Review.
Cited by
-
Breaking the Chain: Protease Inhibitors as Game Changers in Respiratory Viruses Management.Int J Mol Sci. 2024 Jul 25;25(15):8105. doi: 10.3390/ijms25158105. Int J Mol Sci. 2024. PMID: 39125676 Free PMC article. Review.
-
Rapid evolution leads to extensive genetic diversification of cattle flu Influenza D virus.Commun Biol. 2024 Oct 7;7(1):1276. doi: 10.1038/s42003-024-06954-4. Commun Biol. 2024. PMID: 39375524 Free PMC article.
-
RNA virus reverse genetics and vaccine design.Viruses. 2014 Jun 25;6(7):2531-50. doi: 10.3390/v6072531. Viruses. 2014. PMID: 24967693 Free PMC article. Review.
-
The Molecular Epidemiology and Clinical Phylogenetics of Rhinoviruses Among Paediatric Cases in Sydney, Australia.Int J Infect Dis. 2021 Sep;110:69-74. doi: 10.1016/j.ijid.2021.06.046. Epub 2021 Jun 24. Int J Infect Dis. 2021. PMID: 34174431 Free PMC article.
-
Is there still room for novel viral pathogens in pediatric respiratory tract infections?PLoS One. 2014 Nov 20;9(11):e113570. doi: 10.1371/journal.pone.0113570. eCollection 2014. PLoS One. 2014. PMID: 25412469 Free PMC article.
References
-
- Bertino JS (2002) Cost burden of viral respiratory infections: issues for formulary decision makers. Am J Med 112 (Suppl 6A)42S–49S. - PubMed
-
- Fendrick AM, Monto AS, Nightengale B, Sarnes M (2003) The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med 163(4): 487–494. - PubMed
-
- Abzug MJ, Beam AC, Gyorkos EA, Levin MJ (1990) Viral pneumonia in the first month of life. Pediatr Infect Dis J 9(12): 881–885. - PubMed
-
- Stott EJ, Grist NR, Eadie MB (1968) Rhinovirus infections in chronic bronchitis: isolation of eight possibly new Rhinovirus serotypes. J Med Microbio 1(1): 109–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

