Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation
- PMID: 24586479
- PMCID: PMC3930605
- DOI: 10.1371/journal.pone.0089006
Stromal cells from human decidua exert a strong inhibitory effect on NK cell function and dendritic cell differentiation
Abstract
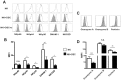
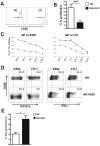
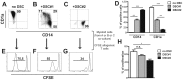
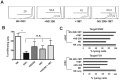
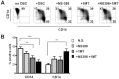
Stromal cells (SC) are an important component of decidual tissues where they are in strict proximity with both NK and CD14(+) myelomonocytic cells that play a role in the maintenance of pregnancy. In this study we analyzed whether decidual SC (DSC) could exert a regulatory role on NK and CD14(+) cells that migrate from peripheral blood (PB) to decidua during pregnancy. We show that DSCs inhibit the IL15-mediated up-regulation of major activating NK receptors in PB-derived NK cells. In addition, the IL15-induced NK cell proliferation, cytolytic activity and IFN-γ production were severely impaired. DSCs sharply inhibited dendritic cells differentiation and their ability to induce allogeneic T cell proliferation. Indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) mediated the inhibitory effect of DSCs. Our results strongly suggest an important role of DSCs in preventing potentially dangerous immune response, thus contributing to maintenance of pregnancy.
Conflict of interest statement
Figures


References
-
- Cerwenka A, Lanier LL (2001) Natural killer cells, viruses and cancer. Nat Rev Immunol 1: 41–49. - PubMed
-
- Le Bouteiller P, Tabiasco J (2006) Killers become builders during pregnancy. Nat Med 12: 991–992. - PubMed
-
- Moretta A (2002) Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol 2: 957–964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

