Inflammation enhances IL-2 driven differentiation of cytolytic CD4 T cells
- PMID: 24586481
- PMCID: PMC3930678
- DOI: 10.1371/journal.pone.0089010
Inflammation enhances IL-2 driven differentiation of cytolytic CD4 T cells
Abstract
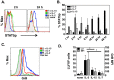
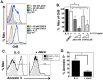
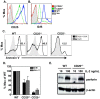
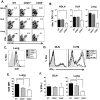
Cytolytic CD4 T cells (CD4 CTL) have been identified in vivo in response to viral infections; however, the factors necessary for driving the cytolytic phenotype have not been fully elucidated. Our previously published work suggests IL-2 may be the master regulator of perforin-mediated cytotoxicity in CD4 effectors. To further dissect the role of IL-2 in CD4 CTL generation, T cell receptor transgenic mice deficient in the ability to produce IL-2 or the high affinity IL-2 receptor (IL-2Rα, CD25) were used. Increasing concentrations of IL-2 were necessary to drive perforin (Prf) expression and maximal cytotoxicity. Granzyme B (GrB) expression and killing correlated with STAT5 activation and CD25 expression in vitro, suggesting that signaling through the high affinity IL-2R is critical for full cytotoxicity. IL-2 signaling was also necessary in vivo for inducing the Th1 phenotype and IFN-γ expression in CD4 T cells during influenza A (IAV) infection. In addition, GrB expression, as measured by mean fluorescent intensity, was decreased in CD25 deficient cells; however, the frequency of CD4 cells expressing GrB was unchanged. Similarly, analysis of cytolytic markers such as CD107a/b and Eomesodermin indicate high IL-2Rα expression is not necessary to drive the CD4 CTL phenotype during IAV infection. Thus, inflammatory signals induced by viral infection may overcome the need for strong IL-2 signals in driving cytotoxicity in CD4 cells.
Conflict of interest statement
Figures


Similar articles
-
The Differentiation and Protective Function of Cytolytic CD4 T Cells in Influenza Infection.Front Immunol. 2016 Mar 9;7:93. doi: 10.3389/fimmu.2016.00093. eCollection 2016. Front Immunol. 2016. PMID: 27014272 Free PMC article. Review.
-
IL-2 and antigen dose differentially regulate perforin- and FasL-mediated cytolytic activity in antigen specific CD4+ T cells.Cell Immunol. 2009;257(1-2):69-79. doi: 10.1016/j.cellimm.2009.03.002. Epub 2009 Mar 31. Cell Immunol. 2009. PMID: 19338979 Free PMC article.
-
Time-Dependent Regulation of IL-2R α-Chain (CD25) Expression by TCR Signal Strength and IL-2-Induced STAT5 Signaling in Activated Human Blood T Lymphocytes.PLoS One. 2016 Dec 9;11(12):e0167215. doi: 10.1371/journal.pone.0167215. eCollection 2016. PLoS One. 2016. PMID: 27936140 Free PMC article.
-
Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells.Immunity. 2010 Jan 29;32(1):79-90. doi: 10.1016/j.immuni.2009.11.012. Epub 2010 Jan 21. Immunity. 2010. PMID: 20096607 Free PMC article.
-
Toward a Molecular Understanding of Adaptive Immunity: A Chronology, Part III.Front Immunol. 2014 Feb 4;5:29. doi: 10.3389/fimmu.2014.00029. eCollection 2014. Front Immunol. 2014. PMID: 24550914 Free PMC article. Review.
Cited by
-
Immunity to Influenza Infection in Humans.Cold Spring Harb Perspect Med. 2021 Mar 1;11(3):a038729. doi: 10.1101/cshperspect.a038729. Cold Spring Harb Perspect Med. 2021. PMID: 31871226 Free PMC article. Review.
-
CTLs heterogeneity and plasticity: implications for cancer immunotherapy.Mol Cancer. 2024 Mar 21;23(1):58. doi: 10.1186/s12943-024-01972-6. Mol Cancer. 2024. PMID: 38515134 Free PMC article. Review.
-
Identification of human cytotoxic ILC3s.Eur J Immunol. 2021 Apr;51(4):811-823. doi: 10.1002/eji.202048696. Epub 2021 Jan 27. Eur J Immunol. 2021. PMID: 33300130 Free PMC article.
-
CD4 CTL, a Cytotoxic Subset of CD4+ T Cells, Their Differentiation and Function.Front Immunol. 2017 Feb 23;8:194. doi: 10.3389/fimmu.2017.00194. eCollection 2017. Front Immunol. 2017. PMID: 28280496 Free PMC article. Review.
-
The Differentiation and Protective Function of Cytolytic CD4 T Cells in Influenza Infection.Front Immunol. 2016 Mar 9;7:93. doi: 10.3389/fimmu.2016.00093. eCollection 2016. Front Immunol. 2016. PMID: 27014272 Free PMC article. Review.
References
-
- Jacobson S, Richert JR, Biddison WE, Satinsky A, Hartzman RJ, et al. (1984) Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol 133: 754–757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

