rtM204Q may serve as a novel lamivudine-resistance-associated mutation of hepatitis B virus
- PMID: 24586482
- PMCID: PMC3933355
- DOI: 10.1371/journal.pone.0089015
rtM204Q may serve as a novel lamivudine-resistance-associated mutation of hepatitis B virus
Abstract
Background and aims: Lamivudine (LAM) is still widely used for anti-HBV therapy in China. The study aimed to clarify whether a newly-found rtM204Q mutation from patients was associated with the drug resistance.
Methods: HBV complete reverse-transcriptase region was screened by direct sequencing and verified by clonal sequencing. Replication-competent plasmids containing patient-derived 1.1mer mutant or wild-type viral genome were constructed and transfected into HepG2 cells. After cultured with or without serially-diluted antiviral drugs, intracellular HBV replicative intermediates were quantitated for calculating the 50% effective concentration of drug (EC₅₀).
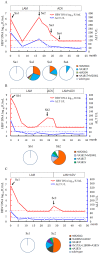
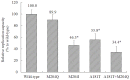
Results: A total of 12,000 serum samples of 9,830 patients with chronic HBV infection were screened. rtM204Q mutation was detected in seven LAM-refractory patients. By contrast, rtM204I/rtM204V mutations were detected in 2,502 patients' samples. The rtM204Q emerged either alone or in concomitance with rtM204I/rtM204V, and all were accompanied with virologic breakthrough in clinical course. Clonal sequencing verified that rtM204Q mutant was predominant in viral quasispecies of these samples. Phenotypic analysis showed that rtM204Q mutant had 89.9% of replication capacity and 76-fold increased LAM EC₅₀ of the concomitant wild-type strain. By contrast, rtM204I mutant in the sample had lower replication capacity and higher LAM resistance (46.3% and 1396-fold increased LAM EC₅₀ of the wild-type strain) compared to rtM204Q mutant. rtM204Q mutant was susceptible to adefovir dipivoxil (ADV) in vitro and ADV/ADV+LAM rescue therapy in clinic.
Conclusion: rtM204Q is suggested to be a novel LAM-resistance-associated mutation. It conferred a moderate resistance with higher competent natural replication capacity compared to rtM204I mutation.
Conflict of interest statement
Figures
References
-
- World Health Organization (2012) Hepatitis B fact sheets. Available: http://www.who.int/mediacentre/factsheets/fs204/en/. Updated on July, 2013. Accessed on January 1, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

