Selective activation of hTRPV1 by N-geranyl cyclopropylcarboxamide, an amiloride-insensitive salt taste enhancer
- PMID: 24586504
- PMCID: PMC3930709
- DOI: 10.1371/journal.pone.0089062
Selective activation of hTRPV1 by N-geranyl cyclopropylcarboxamide, an amiloride-insensitive salt taste enhancer
Abstract
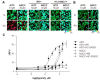
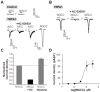
TRPV1t, a variant of the transient receptor potential vanilloid-1 (TRPV1) has been proposed as a constitutively active, non-selective cation channel as a putative amiloride-insensitive salt taste receptor and shares many properties with TRPV1. Based on our previous chorda tympani taste nerve recordings in rodents and human sensory evaluations, we proposed that N-geranylcyclopropylcarboxamide (NGCC), a novel synthetic compound, acts as a salt taste enhancer by modulating the amiloride/benzamil-insensitive Na(+) entry pathways. As an extension of this work, we investigated NGCC-induced human TRPV1 (hTRPV1) activation using a Ca(2+)-flux signaling assay in cultured cells. NGCC enhanced Ca(2+) influx in hTRPV1-expressing cells in a dose-dependent manner (EC50 = 115 µM). NGCC-induced Ca(2+) influx was significantly attenuated by ruthenium red (RR; 30 µM), a non-specific blocker of TRP channels and capsazepine (CZP; 5 µM), a specific antagonist of TRPV1, implying that NGCC directly activates hTRPV1. TRPA1 is often co-expressed with TRPV1 in sensory neurons. Therefore, we also investigated the effects of NGCC on hTRPA1-expressing cells. Similar to hTRPV1, NGCC enhanced Ca(2+) influx in hTRPA1-expressing cells (EC50 = 83.65 µM). The NGCC-induced Ca(2+) influx in hTRPA1-expressing cells was blocked by RR (30 µM) and HC-030031 (100 µM), a specific antagonist of TRPA1. These results suggested that NGCC selectively activates TRPV1 and TRPA1 in cultured cells. These data may provide additional support for our previous hypothesis that NGCC interacts with TRPV1 variant cation channel, a putative amiloride/benzamil-insensitive salt taste pathway in the anterior taste receptive field.
Conflict of interest statement
Figures
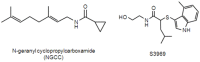




Similar articles
-
N-geranyl cyclopropyl-carboximide modulates salty and umami taste in humans and animal models.J Neurophysiol. 2013 Feb;109(4):1078-90. doi: 10.1152/jn.00124.2012. Epub 2012 Dec 5. J Neurophysiol. 2013. PMID: 23221408 Free PMC article.
-
Regulation of the benzamil-insensitive salt taste receptor by intracellular Ca2+, protein kinase C, and calcineurin.J Neurophysiol. 2009 Sep;102(3):1591-605. doi: 10.1152/jn.91301.2008. Epub 2009 Jun 24. J Neurophysiol. 2009. PMID: 19553475 Free PMC article.
-
Quaternary Lidocaine Derivative QX-314 Activates and Permeates Human TRPV1 and TRPA1 to Produce Inhibition of Sodium Channels and Cytotoxicity.Anesthesiology. 2016 May;124(5):1153-65. doi: 10.1097/ALN.0000000000001050. Anesthesiology. 2016. PMID: 26859646
-
Differential Effect of TRPV1 Modulators on Neural and Behavioral Responses to Taste Stimuli.Nutrients. 2024 Nov 12;16(22):3858. doi: 10.3390/nu16223858. Nutrients. 2024. PMID: 39599644 Free PMC article. Review.
-
Interactions between Chemesthesis and Taste: Role of TRPA1 and TRPV1.Int J Mol Sci. 2021 Mar 25;22(7):3360. doi: 10.3390/ijms22073360. Int J Mol Sci. 2021. PMID: 33806052 Free PMC article. Review.
Cited by
-
Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils.J Leukoc Biol. 2017 Jun;101(6):1361-1371. doi: 10.1189/jlb.3A1216-518RR. Epub 2017 Mar 3. J Leukoc Biol. 2017. PMID: 28258152 Free PMC article.
-
Modulation of Human Neutrophil Responses by the Essential Oils from Ferula akitschkensis and Their Constituents.J Agric Food Chem. 2016 Sep 28;64(38):7156-70. doi: 10.1021/acs.jafc.6b03205. Epub 2016 Sep 13. J Agric Food Chem. 2016. PMID: 27586050 Free PMC article.
-
Kokumi Taste Active Peptides Modulate Salt and Umami Taste.Nutrients. 2020 Apr 24;12(4):1198. doi: 10.3390/nu12041198. Nutrients. 2020. PMID: 32344605 Free PMC article.
-
Enhancement of cellular glucose uptake by reactive species: a promising approach for diabetes therapy.RSC Adv. 2018 Mar 8;8(18):9887-9894. doi: 10.1039/c7ra13389h. eCollection 2018 Mar 5. RSC Adv. 2018. PMID: 35540836 Free PMC article.
-
Urate crystal induced inflammation and joint pain are reduced in transient receptor potential ankyrin 1 deficient mice--potential role for transient receptor potential ankyrin 1 in gout.PLoS One. 2015 Feb 6;10(2):e0117770. doi: 10.1371/journal.pone.0117770. eCollection 2015. PLoS One. 2015. PMID: 25658427 Free PMC article.
References
-
- Mattes RD, Donnelly D (1991) Relative contributions of dietary sodium sources. J Am Coll Nutr 10: 383–393. - PubMed
-
- Karppanen H, Mervaala E (2006) Sodium intake and hypertension. Prog Cardiovasc Dis 49: 59–75. - PubMed
-
- Alexander G, Logan MD (2006) Dietary sodium intake and its relation to human health: a summary of the evidence. J Am Coll Nutr 25: 165–169. - PubMed
-
- Evans CE, Chughtai AY, Blumsohn A, Giles M, Eastell R (1997) The effect of dietary sodium on calcium metabolism in pre menopausal and postmenopausal women. Eur J Clin Nutr 51: 394–399. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

