Zonisamide attenuates α-synuclein neurotoxicity by an aggregation-independent mechanism in a rat model of familial Parkinson's disease
- PMID: 24586512
- PMCID: PMC3930669
- DOI: 10.1371/journal.pone.0089076
Zonisamide attenuates α-synuclein neurotoxicity by an aggregation-independent mechanism in a rat model of familial Parkinson's disease
Abstract
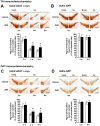
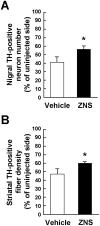
The anti-epileptic agent zonisamide (ZNS) has been shown to exert protective effects in neurotoxin-based mouse models of Parkinson disease. However, it is unknown whether ZNS can attenuate toxicity of familial Parkinson's disease-causing gene products. In this study, we investigated the effects of ZNS on neurodegeneration induced by expression of A53T α-synuclein in the rat substantia nigra using a recombinant adeno-associated virus vector. Expression of A53T α-synuclein yielded severe loss of nigral dopamine neurons and striatal dopamine nerve terminals from 2 weeks to 4 weeks after viral injection. Oral administration of ZNS (40 mg/kg/day) significantly delayed the pace of degeneration at 4 weeks after viral injection as compared with the vehicle group. This effect lasted until 8 weeks after viral injection, the final point of observation. ZNS treatment had no impact on the survival of nigrostriatal dopamine neurons in rats expressing green fluorescent protein. Quantification of striatal Ser129-phosphorylated α-synuclein-positive aggregates showed that these aggregates rapidly formed from 2 weeks to 4 weeks after viral injection. This increase was closely correlated with loss of nigrostriatal dopamine neurons. However, ZNS treatment failed to alter the number of all striatal Ser129-phosphorylated α-synuclein-positive aggregates, including small dot-like and large round structures. The number of these aggregates was almost constant at 4 weeks and 8 weeks after viral injection, although ZNS persistently prevented loss of nigrostriatal dopamine neurons during this period. Also, ZNS treatment did not affect the number of striatal aggregates larger than 10 µm in diameter. These data show that ZNS attenuates α-synuclein-induced toxicity in a manner that is independent of the formation and maturation of α-synuclein aggregates in an in vivo model of familial Parkinson's disease, suggesting that ZNS may protect nigrostriatal dopamine neurons by modulating cellular damage or a cell death pathway commonly caused by neurotoxins and α-synuclein.
Conflict of interest statement
Figures




References
-
- Olanow CW, Watts RL, Koller WC (2001) An algorithm (decision tree) for the management of Parkinson’s disease (2001): treatment guidelines. Neurology 56: S1–S88. - PubMed
-
- Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, et al. (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19: 997–1005. - PubMed
-
- Olanow CW, Jankovic J (2005) Neuroprotective therapy in Parkinson’s disease and motor complications: a search for a pathogenesis-targeted, disease-modifying strategy. Mov Disord 20 Suppl 11S3–10. - PubMed
-
- Olanow CW, Kieburtz K, Schapira AH (2008) Why have we failed to achieve neuroprotection in Parkinson’s disease? Ann Neurol 64 Suppl 2S101–110. - PubMed
-
- Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, et al. (2001) Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol 65: 135–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

