Combining the ABL1 kinase inhibitor ponatinib and the histone deacetylase inhibitor vorinostat: a potential treatment for BCR-ABL-positive leukemia
- PMID: 24586514
- PMCID: PMC3938434
- DOI: 10.1371/journal.pone.0089080
Combining the ABL1 kinase inhibitor ponatinib and the histone deacetylase inhibitor vorinostat: a potential treatment for BCR-ABL-positive leukemia
Abstract
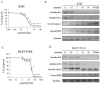
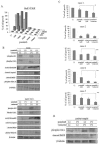
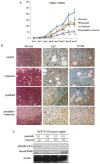
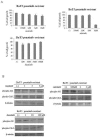
Resistance to imatinib (Gleevec®) in cancer cells is frequently because of acquired point mutations in the kinase domain of BCR-ABL. Ponatinib, also known as AP24534, is an oral multi-targeted tyrosine kinase inhibitor (TKI), and it has been investigated in a pivotal phase 2 clinical trial. The histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid) has been evaluated for its significant clinical activity in hematological malignancies. Thus, treatments combining ABL TKIs with additional drugs may be a promising strategy in the treatment of leukemia. In the current study, we analyzed the efficacy of ponatinib and vorinostat treatment by using BCR-ABL-positive cell lines. Treatment with ponatinib for 72 h inhibited cell growth and induced apoptosis in K562 cells in a dose-dependent manner. We found that ponatinib potently inhibited the growth of Ba/F3 cells ectopically expressing BCR-ABL T315I mutation. Upon BCR-ABL phosphorylation, Crk-L was decreased, and poly (ADP-ribose) polymerase (PARP) was activated in a dose-dependent manner. Combined treatment of Ba/F3 T315I mutant cells with vorinostat and ponatinib resulted in significantly increased cytotoxicity. Additionally, the intracellular signaling of ponatinib and vorinostat was examined. Caspase 3 and PARP activation increased after combination treatment with ponatinib and vorinostat. Moreover, an increase in the phosphorylation levels of γH2A.X was observed. Previously established ponatinib-resistant Ba/F3 cells were also resistant to imatinib, nilotinib, and dasatinib. We investigated the difference in the efficacy of ponatinib and vorinostat by using ponatinib-resistant Ba/F3 cells. Combined treatment of ponatinib-resistant cells with ponatinib and vorinostat caused a significant increase in cytotoxicity. Thus, combined administration of ponatinib and vorinostat may be a powerful strategy against BCR-ABL mutant cells and could enhance the cytotoxic effects of ponatinib in those BCR-ABL mutant cells.
Conflict of interest statement
Figures
Similar articles
-
Combination of panobinostat with ponatinib synergistically overcomes imatinib-resistant CML cells.Cancer Sci. 2016 Jul;107(7):1029-38. doi: 10.1111/cas.12965. Epub 2016 Jun 21. Cancer Sci. 2016. PMID: 27166836 Free PMC article.
-
Effect of dual inhibition of histone deacetylase and phosphatidylinositol-3 kinase in Philadelphia chromosome-positive leukemia cells.Cancer Chemother Pharmacol. 2020 Feb;85(2):401-412. doi: 10.1007/s00280-019-04022-x. Epub 2020 Jan 4. Cancer Chemother Pharmacol. 2020. PMID: 31901955
-
Efficacy of ponatinib against ABL tyrosine kinase inhibitor-resistant leukemia cells.Biochem Biophys Res Commun. 2013 Jun 7;435(3):506-11. doi: 10.1016/j.bbrc.2013.05.022. Epub 2013 May 14. Biochem Biophys Res Commun. 2013. PMID: 23684619
-
Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia.Drugs. 2014 May;74(7):793-806. doi: 10.1007/s40265-014-0216-6. Drugs. 2014. PMID: 24807266 Review.
-
[Is AP24534 (Ponatinib) the next treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia?].Bull Cancer. 2011 Jul;98(7):761-7. doi: 10.1684/bdc.2011.1390. Bull Cancer. 2011. PMID: 21700550 Review. French.
Cited by
-
Non ABL-directed inhibitors as alternative treatment strategies for chronic myeloid leukemia.Mol Cancer. 2018 Feb 19;17(1):56. doi: 10.1186/s12943-018-0805-1. Mol Cancer. 2018. PMID: 29455672 Free PMC article. Review.
-
Combination of panobinostat with ponatinib synergistically overcomes imatinib-resistant CML cells.Cancer Sci. 2016 Jul;107(7):1029-38. doi: 10.1111/cas.12965. Epub 2016 Jun 21. Cancer Sci. 2016. PMID: 27166836 Free PMC article.
-
Management of Chronic Myeloid Leukemia in Advanced Phase.Front Oncol. 2019 Oct 25;9:1132. doi: 10.3389/fonc.2019.01132. eCollection 2019. Front Oncol. 2019. PMID: 31709190 Free PMC article. Review.
-
Thrombotic microangiopathy as a cause of cardiovascular toxicity from the BCR-ABL1 tyrosine kinase inhibitor ponatinib.Blood. 2019 Apr 4;133(14):1597-1606. doi: 10.1182/blood-2018-10-881557. Epub 2019 Jan 28. Blood. 2019. PMID: 30692122 Free PMC article.
-
Effective elimination of chronic lymphocytic leukemia cells in the stromal microenvironment by a novel drug combination strategy using redox-mediated mechanisms.Mol Med Rep. 2015 Nov;12(5):7374-88. doi: 10.3892/mmr.2015.4364. Epub 2015 Sep 25. Mol Med Rep. 2015. PMID: 26458979 Free PMC article.
References
-
- Rowley JD (1973) Letter: A new consistent chromosomal abnormality in chronic myelogenous leukemia identified by quinacrine fluorescence and Giemsa staining. Nature 243: 290–293. - PubMed
-
- Sawyers CL, Callahan W, Witte ON (1992) Dominant negative MYC blocks transformation by ABL oncogenes. Cell 70: 901–910. - PubMed
-
- Ilaria RL Jr, Van Etten RA (1996) P210 and P190 (BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem 271: 31704–31710. - PubMed
-
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, et al. (2002) Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med 346: 645–652. - PubMed
-
- Kantarjian HM, Talpaz M, Giles F, O'Brien S, Cortes J (2006) New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance,. Ann Intern Med 145: 913–923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

