A compartmentalized mathematical model of the β1-adrenergic signaling system in mouse ventricular myocytes
- PMID: 24586529
- PMCID: PMC3931689
- DOI: 10.1371/journal.pone.0089113
A compartmentalized mathematical model of the β1-adrenergic signaling system in mouse ventricular myocytes
Abstract
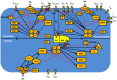
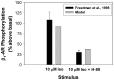
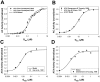
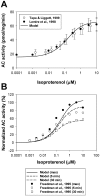
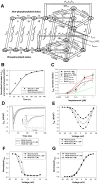
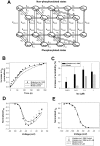
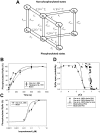
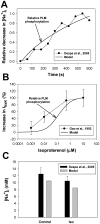
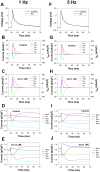
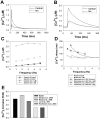
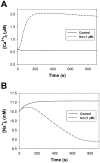
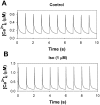
The β1-adrenergic signaling system plays an important role in the functioning of cardiac cells. Experimental data shows that the activation of this system produces inotropy, lusitropy, and chronotropy in the heart, such as increased magnitude and relaxation rates of [Ca(2+)]i transients and contraction force, and increased heart rhythm. However, excessive stimulation of β1-adrenergic receptors leads to heart dysfunction and heart failure. In this paper, a comprehensive, experimentally based mathematical model of the β1-adrenergic signaling system for mouse ventricular myocytes is developed, which includes major subcellular functional compartments (caveolae, extracaveolae, and cytosol). The model describes biochemical reactions that occur during stimulation of β1-adrenoceptors, changes in ionic currents, and modifications of Ca(2+) handling system. Simulations describe the dynamics of major signaling molecules, such as cyclic AMP and protein kinase A, in different subcellular compartments; the effects of inhibition of phosphodiesterases on cAMP production; kinetics and magnitudes of phosphorylation of ion channels, transporters, and Ca(2+) handling proteins; modifications of action potential shape and duration; magnitudes and relaxation rates of [Ca(2+)]i transients; changes in intracellular and transmembrane Ca(2+) fluxes; and [Na(+)]i fluxes and dynamics. The model elucidates complex interactions of ionic currents upon activation of β1-adrenoceptors at different stimulation frequencies, which ultimately lead to a relatively modest increase in action potential duration and significant increase in [Ca(2+)]i transients. In particular, the model includes two subpopulations of the L-type Ca(2+) channels, in caveolae and extracaveolae compartments, and their effects on the action potential and [Ca(2+)]i transients are investigated. The presented model can be used by researchers for the interpretation of experimental data and for the developments of mathematical models for other species or for pathological conditions.
Conflict of interest statement
Figures

Similar articles
-
A compartmentalized mathematical model of mouse atrial myocytes.Am J Physiol Heart Circ Physiol. 2020 Mar 1;318(3):H485-H507. doi: 10.1152/ajpheart.00460.2019. Epub 2020 Jan 17. Am J Physiol Heart Circ Physiol. 2020. PMID: 31951471 Free PMC article.
-
Distinct physiological effects of β1- and β2-adrenoceptors in mouse ventricular myocytes: insights from a compartmentalized mathematical model.Am J Physiol Cell Physiol. 2017 May 1;312(5):C595-C623. doi: 10.1152/ajpcell.00273.2016. Epub 2017 Jan 25. Am J Physiol Cell Physiol. 2017. PMID: 28122730
-
Simulation of the effects of moderate stimulation/inhibition of the β1-adrenergic signaling system and its components in mouse ventricular myocytes.Am J Physiol Cell Physiol. 2016 Jun 1;310(11):C844-56. doi: 10.1152/ajpcell.00002.2016. Epub 2016 Mar 2. Am J Physiol Cell Physiol. 2016. PMID: 26936457
-
Mathematical modeling physiological effects of the overexpression of β2-adrenoceptors in mouse ventricular myocytes.Am J Physiol Heart Circ Physiol. 2018 Mar 1;314(3):H643-H658. doi: 10.1152/ajpheart.00160.2017. Epub 2017 Nov 3. Am J Physiol Heart Circ Physiol. 2018. PMID: 29101164
-
Suppression of lusitropy as a disease mechanism in cardiomyopathies.Front Cardiovasc Med. 2023 Jan 9;9:1080965. doi: 10.3389/fcvm.2022.1080965. eCollection 2022. Front Cardiovasc Med. 2023. PMID: 36698941 Free PMC article. Review.
Cited by
-
Optogenetic stimulation of Gs-signaling in the heart with high spatio-temporal precision.Nat Commun. 2019 Mar 20;10(1):1281. doi: 10.1038/s41467-019-09322-7. Nat Commun. 2019. PMID: 30894542 Free PMC article.
-
Investigating β-adrenergic-induced cardiac hypertrophy through computational approach: classical and non-classical pathways.J Physiol Sci. 2018 Jul;68(4):503-520. doi: 10.1007/s12576-017-0557-5. Epub 2017 Jul 3. J Physiol Sci. 2018. PMID: 28674776 Free PMC article.
-
Quantitative cross-species translators of cardiac myocyte electrophysiology: Model training, experimental validation, and applications.Sci Adv. 2021 Nov 19;7(47):eabg0927. doi: 10.1126/sciadv.abg0927. Epub 2021 Nov 17. Sci Adv. 2021. PMID: 34788089 Free PMC article.
-
A compartmentalized mathematical model of mouse atrial myocytes.Am J Physiol Heart Circ Physiol. 2020 Mar 1;318(3):H485-H507. doi: 10.1152/ajpheart.00460.2019. Epub 2020 Jan 17. Am J Physiol Heart Circ Physiol. 2020. PMID: 31951471 Free PMC article.
-
Molecular noise filtering in the β-adrenergic signaling network by phospholamban pentamers.Cell Rep. 2021 Jul 27;36(4):109448. doi: 10.1016/j.celrep.2021.109448. Cell Rep. 2021. PMID: 34320358 Free PMC article.
References
-
- Schaub MC, Hefti MA, Zaugg M (2006) Integration of calcium with the signaling network in cardiac myocytes. J Mol Cell Cardiol 41: 183–214. - PubMed
-
- Wachter SB, Gilbert EM (2012) Beta-adrenergic receptors, from their discovery and characterization through their manipulation to beneficial clinical application. Cardiology 122: 104–112. - PubMed
-
- Reincober J, Tscheschner H, Pleger ST, Most P, Katus HA, et al. (2012) Targeting GRK2 by gene therapy for heart failure: benefits above β-blockade. Gene Ther 19: 686–693. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

