Women's experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa
- PMID: 24586534
- PMCID: PMC3931679
- DOI: 10.1371/journal.pone.0089118
Women's experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa
Abstract
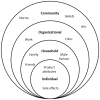
Background: In VOICE, a multisite HIV pre-exposure prophylaxis (PrEP) trial, plasma drug levels pointed to widespread product nonuse, despite high adherence estimated by self-reports and clinic product counts. Using a socio-ecological framework (SEF), we explored socio-cultural and contextual factors that influenced participants' experience of daily vaginal gel and oral tablet regimens in VOICE.
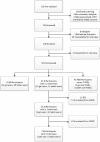
Methods: In Johannesburg, a qualitative ancillary study was concurrently conducted among randomly selected VOICE participants assigned to in-depth interviews (n = 41), serial ethnographic interviews (n = 21), or focus group discussions (n = 40). Audiotaped interviews were transcribed, translated, and coded thematically for analysis.
Results: Of the 102 participants, the mean age was 27 years, and 96% had a primary sex partner with whom 43% cohabitated. Few women reported lasting nonuse, which they typically attributed to missed visits, lack of product replenishments, and family-related travel or work. Women acknowledged occasionally skipping or mistiming doses because they forgot, were busy, felt lazy or bored, feared or experienced side effects. However, nearly all knew or heard of other study participants who did not use products daily. Three overarching themes emerged from further analyses: ambivalence toward research, preserving a healthy status, and managing social relationships. These themes highlighted the profound and complex meanings associated with participating in a blinded HIV PrEP trial and taking antiretroviral-based products. The unknown efficacy of products, their connection with HIV infection, challenges with daily regimen given social risks, lack of support-from partners and significant others-and the relationship tradeoffs entailed by using the products appear to discourage adequate product use.
Conclusions: Personal acknowledgment of product nonuse was challenging. This qualitative inquiry highlighted key influences at all SEF levels that shaped women's perceptions of trial participation and experiences with investigational products. Whether these impacted women's behaviors and may have contributed to ineffective trial results warrants further investigation.
Conflict of interest statement
Figures
References
-
- Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, et al.. (2012) Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med doi:10.1056/NEJMoa1110711: 1–12. - PubMed
-
- Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, et al. (2013) Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet 381: 2083–2090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

