Twisted gastrulation, a BMP antagonist, exacerbates podocyte injury
- PMID: 24586548
- PMCID: PMC3934867
- DOI: 10.1371/journal.pone.0089135
Twisted gastrulation, a BMP antagonist, exacerbates podocyte injury
Abstract
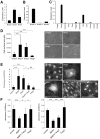
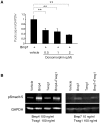
Podocyte injury is the first step in the progression of glomerulosclerosis. Previous studies have demonstrated the beneficial effect of bone morphogenetic protein 7 (Bmp7) in podocyte injury and the existence of native Bmp signaling in podocytes. Local activity of Bmp7 is controlled by cell-type specific Bmp antagonists, which inhibit the binding of Bmp7 to its receptors. Here we show that the product of Twisted gastrulation (Twsg1), a Bmp antagonist, is the central negative regulator of Bmp function in podocytes and that Twsg1 null mice are resistant to podocyte injury. Twsg1 was the most abundant Bmp antagonist in murine cultured podocytes. The administration of Bmp induced podocyte differentiation through Smad signaling, whereas the simultaneous administration of Twsg1 antagonized the effect. The administration of Bmp also inhibited podocyte proliferation, whereas simultaneous administration of Twsg1 antagonized the effect. Twsg1 was expressed in the glomerular parietal cells (PECs) and distal nephron of the healthy kidney, and additionally in damaged glomerular cells in a murine model of podocyte injury. Twsg1 null mice exhibited milder hypoalbuminemia and hyperlipidemia, and milder histological changes while maintaining the expression of podocyte markers during podocyte injury model. Taken together, our results show that Twsg1 plays a critical role in the modulation of protective action of Bmp7 on podocytes, and that inhibition of Twsg1 is a promising means of development of novel treatment for podocyte injury.
Conflict of interest statement
Figures


References
-
- LeHir M, Kriz W (2007) New insights into structural patterns encountered in glomerulosclerosis. Curr Opin Nephrol Hypertens 16: 184–191. - PubMed
-
- D'Agati VD (2008) Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406. - PubMed
-
- Wiggins RC (2007) The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214. - PubMed
-
- Barisoni L, Schnaper HW, Kopp JB (2007) A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542. - PubMed
-
- Mundel P, Shankland SJ (2002) Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

