Deletion of PREPl causes growth impairment and hypotonia in mice
- PMID: 24586561
- PMCID: PMC3938459
- DOI: 10.1371/journal.pone.0089160
Deletion of PREPl causes growth impairment and hypotonia in mice
Abstract
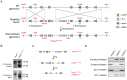
Genetic studies of rare diseases can identify genes of unknown function that strongly impact human physiology. Prolyl endopeptidase-like (PREPL) is an uncharacterized member of the prolyl peptidase family that was discovered because of its deletion in humans with hypotonia-cystinuria syndrome (HCS). HCS is characterized by a number of physiological changes including diminished growth and neonatal hypotonia or low muscle tone. HCS patients have deletions in other genes as well, making it difficult to tease apart the specific role of PREPL. Here, we develop a PREPL null (PREPL(-/-)) mouse model to address the physiological role of this enzyme. Deletion of exon 11 from the Prepl gene, which encodes key catalytic amino acids, leads to a loss of PREPL protein as well as lower Prepl mRNA levels. PREPL(-/-) mice have a pronounced growth phenotype, being significantly shorter and lighter than their wild type (PREPL(+/+)) counterparts. A righting assay revealed that PREPL(-/-) pups took significantly longer than PREPL(+/+) pups to right themselves when placed on their backs. This deficit indicates that PREPL(-/-) mice suffer from neonatal hypotonia. According to these results, PREPL regulates growth and neonatal hypotonia in mice, which supports the idea that PREPL causes diminished growth and neonatal hypotonia in humans with HCS. These animals provide a valuable asset in deciphering the underlying biochemical, cellular and physiological pathways that link PREPL to HCS, and this may eventually lead to new insights in the treatment of this disease.
Conflict of interest statement
Figures



References
-
- Parvari R, Gonen Y, Alshafee I, Buriakovsky S, Regev K, et al. (2005) The 2p21 deletion syndrome: characterization of the transcription content. Genomics 86: 195–211. - PubMed
-
- Martens K, Heulens I, Meulemans S, Zaffanello M, Tilstra D, et al. (2007) Global distribution of the most prevalent deletions causing hypotonia-cystinuria syndrome. European Journal of Human Genetics 15: 1029–1033. - PubMed
-
- Regal L, Aydin HI, Dieltjens A-M, Van Esch H, Francois I, et al. (2012) Two novel deletions in hypotonia-cystinuria syndrome. Molecular genetics and metabolism 107: 614–616. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

