Novel CoQ10 antidiabetic mechanisms underlie its positive effect: modulation of insulin and adiponectine receptors, Tyrosine kinase, PI3K, glucose transporters, sRAGE and visfatin in insulin resistant/diabetic rats
- PMID: 24586567
- PMCID: PMC3930675
- DOI: 10.1371/journal.pone.0089169
Novel CoQ10 antidiabetic mechanisms underlie its positive effect: modulation of insulin and adiponectine receptors, Tyrosine kinase, PI3K, glucose transporters, sRAGE and visfatin in insulin resistant/diabetic rats
Abstract
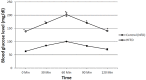
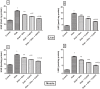
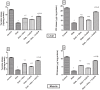
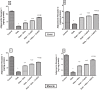
As a nutritional supplement, coenzyme Q10 (CoQ10) was tested previously in several models of diabetes and/or insulin resistance (IR); however, its exact mechanisms have not been profoundly explicated. Hence, the objective of this work is to verify some of the possible mechanisms that underlie its therapeutic efficacy. Moreover, the study aimed to assess the potential modulatory effect of CoQ10 on the antidiabetic action of glimebiride. An insulin resistance/type 2 diabetic model was adopted, in which rats were fed high fat/high fructose diet (HFFD) for 6 weeks followed by a single sub-diabetogenic dose of streptozotocin (35 mg/kg, i.p.). At the end of the 7(th) week animals were treated with CoQ10 (20 mg/kg, p.o) and/or glimebiride (0.5 mg/kg, p.o) for 2 weeks. CoQ10 alone opposed the HFFD effect and increased the hepatic/muscular content/activity of tyrosine kinase (TK), phosphatidylinositol kinase (PI3K), and adiponectin receptors. Conversely, it decreased the content/activity of insulin receptor isoforms, myeloperoxidase and glucose transporters (GLUT4; 2). Besides, it lowered significantly the serum levels of glucose, insulin, fructosamine and HOMA index, improved the serum lipid panel and elevated the levels of glutathione, sRAGE and adiponectin. On the other hand, CoQ10 lowered the serum levels of malondialdehyde, visfatin, ALT and AST. Surprisingly, CoQ10 effect surpassed that of glimepiride in almost all the assessed parameters, except for glucose, fructosamine, TK, PI3K, and GLUT4. Combining CoQ10 with glimepiride enhanced the effect of the latter on the aforementioned parameters.
Conclusion: These results provided a new insight into the possible mechanisms by which CoQ10 improves insulin sensitivity and adjusts type 2 diabetic disorder. These mechanisms involve modulation of insulin and adiponectin receptors, as well as TK, PI3K, glucose transporters, besides improving lipid profile, redox system, sRAGE, and adipocytokines. The study also points to the potential positive effect of CoQ10 as an adds- on to conventional antidiabetic therapies.
Conflict of interest statement
Figures
References
-
- Keller KB, Lemberg L (2003) Obesity and the metabolic syndrome. Am J Crit Care. 12: 167–170. - PubMed
-
- Schaalan M, El-Abhar H, Barakat M, El-Denshary ES (2009) Westernized-like-diet-fed rats: effect on glucose homeostasis, lipid profile, and adipocyte hormones and their modulation by rosiglitazone and glimepiride. J Diabetes Complications 23: 199–208. - PubMed
-
- Lamson DW, Plaza SM (2002) Mitochondrial factors in the pathogenesis of diabetes: a hypothesis for treatment. Altern Med Rev 7: 94–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

