Effect of bodily fluids from honey bee (Apis mellifera) larvae on growth and genome-wide transcriptional response of the causal agent of American Foulbrood disease (Paenibacillus larvae)
- PMID: 24586572
- PMCID: PMC3930689
- DOI: 10.1371/journal.pone.0089175
Effect of bodily fluids from honey bee (Apis mellifera) larvae on growth and genome-wide transcriptional response of the causal agent of American Foulbrood disease (Paenibacillus larvae)
Abstract
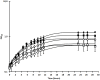
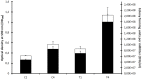
Paenibacillus larvae, the causal agent of American Foulbrood disease (AFB), affects honey bee health worldwide. The present study investigates the effect of bodily fluids from honey bee larvae on growth velocity and transcription for this Gram-positive, endospore-forming bacterium. It was observed that larval fluids accelerate the growth and lead to higher bacterial densities during stationary phase. The genome-wide transcriptional response of in vitro cultures of P. larvae to larval fluids was studied by microarray technology. Early responses of P. larvae to larval fluids are characterized by a general down-regulation of oligopeptide and sugar transporter genes, as well as by amino acid and carbohydrate metabolic genes, among others. Late responses are dominated by general down-regulation of sporulation genes and up-regulation of phage-related genes. A theoretical mechanism of carbon catabolite repression is discussed.
Conflict of interest statement
Figures



References
-
- Ellis JD, Munn PA (2005) The worldwide health status of honey bees. Bee World 86: 88–101.
-
- Crailsheim K, Riessberger-Galle U (2001) Honey bee age-dependent resistance against American foulbrood. Apidologie 32: 91–103.
-
- Brødsgaard CJ, Ritter W, Hansen H (1998) Response of in vitro reared honey bee larvae to various doses of Paenibacillus larvae larvae spores. Apidologie 29: 569–578.
-
- Yue D, Nordhoff M, Wieler LH, Genersch E (2008) Fluorescence in situ hybridization (FISH) analysis of the interactions between honey bee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honey bees (Apis mellifera). Environ Microbiol 10: 1612–1620. - PubMed
-
- Sturtevant AP (1932) Relation of commercial honey to the spread of American foulbrood. J Agricul Res 45: 257–285.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

