Growth differentiation factor 6 as a putative risk factor in neuromuscular degeneration
- PMID: 24586579
- PMCID: PMC3938462
- DOI: 10.1371/journal.pone.0089183
Growth differentiation factor 6 as a putative risk factor in neuromuscular degeneration
Abstract
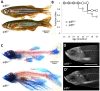
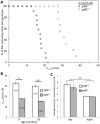
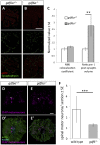
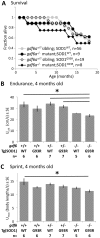
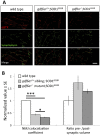
Mutation of Glass bottom boat, the Drosophila homologue of the bone morphogenetic protein or growth/differentiation factor (BMP/GDF) family of genes in vertebrates, has been shown to disrupt development of neuromuscular junctions (NMJ). Here we tested whether this same conclusion can be broadened to vertebrate BMP/GDF genes. This analysis was also extended to consider whether such genes are required for NMJ maintenance in post-larval stages, as this would argue that BMP genes are viable candidates for analysis in progressive neuromuscular disease. Zebrafish mutants harboring homozygous null mutations in the BMP-family gene gdf6a were raised to adulthood and assessed for neuromuscular deficits. Fish lacking gdf6a exhibited decreased endurance (∼ 50%, p = 0.005) compared to wild type, and this deficit progressively worsened with age. These fish also presented with significantly disrupted NMJ morphology (p = 0.009), and a lower abundance of spinal motor neurons (∼ 50%, p<0.001) compared to wild type. Noting the similarity of these symptoms to those of Amyotrophic Lateral Sclerosis (ALS) model mice and fish, we asked if mutations in gdf6a would enhance the phenotypes observed in the latter, i.e. in zebrafish over-expressing mutant Superoxide Dismutase 1 (SOD1). Amongst younger adult fish only bigenic fish harboring both the SOD1 transgene and gdf6a mutations, but not siblings with other combinations of these gene modifications, displayed significantly reduced endurance (75%, p<0.05) and strength/power (75%, p<0.05), as well as disrupted NMJ morphology (p<0.001) compared to wild type siblings. Bigenic fish also had lower survival rates compared to other genotypes. Thus conclusions regarding a role for BMP ligands in effecting NMJ can be extended to vertebrates, supporting conservation of mechanisms relevant to neuromuscular degenerative diseases. These conclusions synergize with past findings to argue for further analysis of GDF6 and other BMP genes as modifier loci, potentially affecting susceptibility to ALS and perhaps a broader suite of neurodegenerative diseases.
Conflict of interest statement
Figures
References
-
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, et al. (2003) The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39: 241–254. - PubMed
-
- Ruschke K, Hiepen C, Becker J, Knaus P (2012) BMPs are mediators in tissue crosstalk of the regenerating musculoskeletal system. Cell Tissue Res 347: 521–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

