Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis
- PMID: 24586612
- PMCID: PMC3931710
- DOI: 10.1371/journal.pone.0089227
Periadventitial application of rapamycin-loaded nanoparticles produces sustained inhibition of vascular restenosis
Abstract
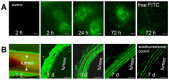
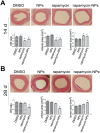
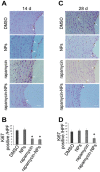
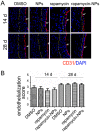
Open vascular reconstructions frequently fail due to the development of recurrent disease or intimal hyperplasia (IH). This paper reports a novel drug delivery method using a rapamycin-loaded poly(lactide-co-glycolide) (PLGA) nanoparticles (NPs)/pluronic gel system that can be applied periadventitially around the carotid artery immediately following the open surgery. In vitro studies revealed that rapamycin dispersed in pluronic gel was rapidly released over 3 days whereas release of rapamycin from rapamycin-loaded PLGA NPs embedded in pluronic gel was more gradual over 4 weeks. In cultured rat vascular smooth muscle cells (SMCs), rapamycin-loaded NPs produced durable (14 days versus 3 days for free rapamycin) inhibition of phosphorylation of S6 kinase (S6K1), a downstream target in the mTOR pathway. In a rat balloon injury model, periadventitial delivery of rapamycin-loaded NPs produced inhibition of phospho-S6K1 14 days after balloon injury. Immunostaining revealed that rapamycin-loaded NPs reduced SMC proliferation at both 14 and 28 days whereas rapamycin alone suppressed proliferation at day 14 only. Moreover, rapamycin-loaded NPs sustainably suppressed IH for at least 28 days following treatment, whereas rapamycin alone produced suppression on day 14 with rebound of IH by day 28. Since rapamycin, PLGA, and pluronic gel have all been approved by the FDA for other human therapies, this drug delivery method could potentially be translated into human use quickly to prevent failure of open vascular reconstructions.
Conflict of interest statement
Figures


Similar articles
-
A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia.J Control Release. 2014 Oct 10;191:47-53. doi: 10.1016/j.jconrel.2014.05.017. Epub 2014 May 20. J Control Release. 2014. PMID: 24852098 Free PMC article.
-
Rapamycin-loaded nanoparticles for inhibition of neointimal hyperplasia in experimental vein grafts.Ann Vasc Surg. 2011 May;25(4):538-46. doi: 10.1016/j.avsg.2011.01.003. Ann Vasc Surg. 2011. PMID: 21549923
-
Intraluminal Delivery of Simvastatin Attenuates Intimal Hyperplasia After Arterial Injury.Vasc Endovascular Surg. 2019 Jul;53(5):379-386. doi: 10.1177/1538574419833224. Epub 2019 Apr 14. Vasc Endovascular Surg. 2019. PMID: 30982448
-
Rapamycin-loaded nanoparticles for inhibition of neointimal hyperplasia in experimental vein grafts.J Cardiothorac Surg. 2011 May 12;6:69. doi: 10.1186/1749-8090-6-69. J Cardiothorac Surg. 2011. Retraction in: J Cardiothorac Surg. 2012 Mar 06;7:17. doi: 10.1186/1749-8090-7-17. PMID: 21569412 Free PMC article. Retracted.
-
Periadventitial drug delivery for the prevention of intimal hyperplasia following open surgery.J Control Release. 2016 Jul 10;233:174-80. doi: 10.1016/j.jconrel.2016.05.002. Epub 2016 May 12. J Control Release. 2016. PMID: 27179635 Free PMC article. Review.
Cited by
-
A rapamycin-releasing perivascular polymeric sheath produces highly effective inhibition of intimal hyperplasia.J Control Release. 2014 Oct 10;191:47-53. doi: 10.1016/j.jconrel.2014.05.017. Epub 2014 May 20. J Control Release. 2014. PMID: 24852098 Free PMC article.
-
A Biodegradable Microneedle Cuff for Comparison of Drug Effects through Perivascular Delivery to Balloon-Injured Arteries.Polymers (Basel). 2017 Feb 8;9(2):56. doi: 10.3390/polym9020056. Polymers (Basel). 2017. PMID: 30970733 Free PMC article.
-
Restenosis Inhibition and Re-differentiation of TGFβ/Smad3-activated Smooth Muscle Cells by Resveratrol.Sci Rep. 2017 Feb 6;7:41916. doi: 10.1038/srep41916. Sci Rep. 2017. PMID: 28165488 Free PMC article.
-
Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging.Adv Funct Mater. 2017 Feb 23;27(8):1604671. doi: 10.1002/adfm.201604671. Epub 2017 Jan 17. Adv Funct Mater. 2017. PMID: 28989337 Free PMC article.
-
Existing and Evolving Therapies for Arteriovenous Fistula and Graft Dysfunction.Indian J Nephrol. 2024 Nov-Dec;34(6):552-560. doi: 10.25259/ijn_528_23. Epub 2024 Jul 1. Indian J Nephrol. 2024. PMID: 39649313 Free PMC article. Review.
References
-
- Curcio A, Torella D, Indolfi C (2011) Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy. Circ J 75: 1287–1296. - PubMed
-
- Acharya S, Sahoo SK (2011) PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev 63: 170–183. - PubMed
-
- Martin-Banderas L, Durán-Lobato M, Muñoz-Rubio I, Alvarez-Fuentes J, Fernández-Arevalo M, et al. (2013) Functional PLGA NPs for oral drug delivery: recent strategies and developments. Mini Rev Med Chem 13: 58–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

