Conditional deletion of ferritin h in mice reduces B and T lymphocyte populations
- PMID: 24586648
- PMCID: PMC3931725
- DOI: 10.1371/journal.pone.0089270
Conditional deletion of ferritin h in mice reduces B and T lymphocyte populations
Abstract
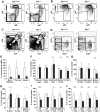
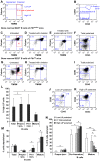
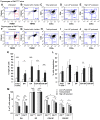
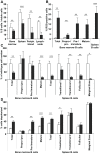
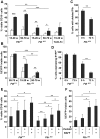
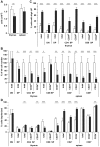
The immune system and iron availability are intimately linked as appropriate iron supply is needed for cell proliferation, while excess iron, as observed in hemochromatosis, may reduce subsets of lymphocytes. We have tested the effects of a ferritin H gene deletion on lymphocytes. Mx-Cre mediated conditional deletion of ferritin H in bone marrow reduced the number of mature B cells and peripheral T cells in all lymphoid organs. FACS analysis showed an increase in the labile iron pool, enhanced reactive oxygen species formation and mitochondrial depolarization. The findings were confirmed by a B-cell specific deletion using Fth(lox/lox) ; CD19-Cre mice. Mature B cells were strongly under-represented in bone marrow and spleen of the deleted mice, whereas pre-B and immature B cells were not affected. Bone marrow B cells showed increased proliferation as judged by the number of cells in S and G2/M phase as well as BrdU incorporation. Upon in vitro culture with B-cell activating factor of the tumor necrosis factor family (BAFF), ferritin H-deleted spleen B cells showed lower survival rates than wild type cells. This was partially reversed with iron-chelator deferiprone. The loss of T cells was also confirmed by a T cell-specific deletion in Fth(lox/lox) ;CD4-Cre mice. Our data show that ferritin H is required for B and T cell survival by actively reducing the labile iron pool. They further suggest that natural B and T cell maturation is influenced by intracellular iron levels and possibly deregulated in iron excess or deprivation.
Conflict of interest statement
Figures

References
-
- Kakhlon O, Gruenbaum Y, Cabantchik ZL (2001) Repression of ferritin expression increases the labile iron pool, oxidative stress, and short-term growth of human erythroleukemia cells. Blood 97: 2863–2871. - PubMed
-
- Kakhlon O, Cabantchik ZI (2002) The labile iron pool: Characterization, measurement, and participation in cellular processes. Free Radic Biol Med 33: 1037–1046. - PubMed
-
- Hentze MW, Muckenthaler MU, Andrews NC (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297. - PubMed
-
- Kühn LC (2009) How iron controls iron. Cell Metab 10: 439–441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

