Feasibility of decentralised deployment of Xpert MTB/RIF test at lower level of health system in India
- PMID: 24586675
- PMCID: PMC3935858
- DOI: 10.1371/journal.pone.0089301
Feasibility of decentralised deployment of Xpert MTB/RIF test at lower level of health system in India
Abstract
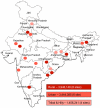
Background: Xpert MTB/RIF is an automated cartridge-based nucleic acid amplification test that has demonstrated its potential to detect tuberculosis and rifampicin resistance with high accuracy. To assist scale-up decisions in India, a feasibility assessment of Xpert MTB/RIF implementation was conducted within microscopy centres of 18 RNTCP TB units.
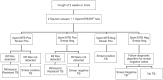
Methods: As part of programme-based demonstration of Xpert MTB/RIF implementation, we recorded and analysed association between key implementation factors and the ability of test to produce valid results. Factors contributing to test failures were analysed from GeneXpert software data which provides 'failure codes' and causes for test failures.
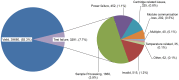
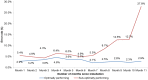
Results: From March'12 to January'13, total 40,035 suspects were tested by Xpert MTB/RIF, and 39,680 (99.1%) received valid results (Cumulative: 37157 (92.8%) on first attempt, 39410 (98.4%) on second attempt, 39637 (99.0%) on third attempt and 39680 (99.1%) on more attempts). Overall initial test failure was 2,878 (7.2% (4%-17%)); of these, 2,594 (90.1%) were re-tested and produced valid results. Most frequent reason of test failure was inadequate sample processing or equipment malfunction (3.9%). Other reasons included power failure (1.1%), cartridge integrity/component failure (0.8%), device-computer communication error (0.5%), and temperature-related errors (0.08%). Significant variation was observed in failure rates both across instruments and over time; furthermore, substantial variation was observed in failure rate in two cartridges lots.
Conclusion: Installation required minimal infrastructure modifications and concerns about adequacy of human resources under public sector facilities and temperature extremes proved unfounded. Under routine conditions, Xpert MTB/RIF provided 99.1% valid results in TB suspects with low overall failure rates (7.2% initial failure, 0.9% final failure); devices provided valuable real-time feedback on reasons for test failure, which were used for rapid corrective action. High modular replacement (32%) and inter-lot cartridge performance variation remain sources of concern, and warrant close monitoring of failure rates as a key quality indicator.
Conflict of interest statement
Figures
References
-
- World Health Organization The Stop TB Department (2010) Roadmap for rolling out Xpert MTB/RIF for rapid diagnosis of TB and MDR-TB.
-
- World Health Organization (2010) Frequently Asked Questions on Xpert MTB/RIF assay: 1–5.
-
- Evans CA (2011) GeneXpert — A Game-Changer for Tuberculosis Control? PLoS Med 8: 8–11 doi: 10.1371/journal.pmed.1001061 . Available: 10.1371/journal.pmed.1001061http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3144196/Accessed. Available: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3144196/Accessed on 24 May 2013 - DOI - PMC - PubMed
-
- World Health Organization (2011) Rapid Implementation of the Xpert MTB/RIF diagnostic test Technical and Operational ‘How – to’; Practical consideration. Available: http://whqlibdoc.who.int/publications/2011/9789241501569_eng.pdf.
-
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, et al. (2010) Rapid Molecular Detection of Tuberculosis and Rifampin Resistance. N Engl J Med 363: 1005–1015 Available: http://www.nejm.org/doi/pdf/10.1056/NEJMoa0907847. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials