Difficulties in eliminating measles and controlling rubella and mumps: a cross-sectional study of a first measles and rubella vaccination and a second measles, mumps, and rubella vaccination
- PMID: 24586717
- PMCID: PMC3930734
- DOI: 10.1371/journal.pone.0089361
Difficulties in eliminating measles and controlling rubella and mumps: a cross-sectional study of a first measles and rubella vaccination and a second measles, mumps, and rubella vaccination
Abstract
Background: The reported coverage of the measles-rubella (MR) or measles-mumps-rubella (MMR) vaccine is greater than 99.0% in Zhejiang province. However, the incidence of measles, mumps, and rubella remains high. In this study, we assessed MMR seropositivity and disease distribution by age on the basis of the current vaccination program, wherein the first dose of MR is administered at 8 months and the second dose of MMR is administered at 18-24 months.
Methods: Cross-sectional serological surveys of MMR antibodies were conducted by collecting epidemiological data in Zhejiang province, China in 2011. In total, 1015 participants were randomly selected from two surveillance sites. Serum MMR-specific immunoglobulin G levels were tested by enzyme-linked immunosorbent assay. The geometric mean titers and seroprevalence with 95% confidence intervals (CIs) were calculated by age and gender. Proportions of different dose of vaccine by age by vaccine were also identified. Statistically significant differences between categories were assessed by the Chi-square test.
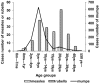
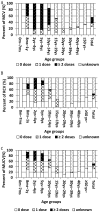
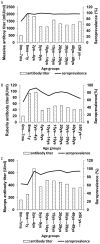
Results: Over 95% seroprevalence rates of measles were seen in all age groups except <7 months infants. Children aged 5-9 years were shown lower seropositivity rates of mumps while elder adolescences and young adults were presented lower rubella seroprevalence. Especially, rubella seropositivity was significantly lower in female adults than in male. Nine measles cases were unvaccinated or unknown vaccination history. Among them, 66.67% (6/9) patients were aged 20-29 years while 33.33% (3/9) were infants aged 8-12 months. In addition, 57.75% (648/1122) patients with mumps were children aged 5-9 years, and 50.54% (94/186) rubella cases were aged 15-39 years.
Conclusions: A timely two-dose MMR vaccination schedule is recommended, with the first dose at 8 months and the second dose at 18-24 months. An MR vaccination speed-up campaign may be necessary for elder adolescents and young adults, particularly young females.
Conflict of interest statement
Figures
Similar articles
-
Age-stratified seroprevalence of measles, mumps and rubella (MMR) virus infections in Switzerland after the introduction of MMR mass vaccination.Eur J Epidemiol. 1997 Jan;13(1):61-6. doi: 10.1023/a:1007326621525. Eur J Epidemiol. 1997. PMID: 9062781
-
Seroprevalence of Measles, Mumps, and Rubella Antibodies in College Students in Mumbai, India.Viral Immunol. 2016 Apr;29(3):159-63. doi: 10.1089/vim.2015.0070. Epub 2016 Feb 24. Viral Immunol. 2016. PMID: 26910764
-
[Measles, mumps and rubella: vaccination rate and seroprevalence in 8th grade students of 8 different sites in Switzerland 1995/96].Praxis (Bern 1994). 1999 Jun 10;88(24):1069-77. Praxis (Bern 1994). 1999. PMID: 10420798 German.
-
Measles, mumps, rubella vaccine (Priorix; GSK-MMR): a review of its use in the prevention of measles, mumps and rubella.Drugs. 2003;63(19):2107-26. doi: 10.2165/00003495-200363190-00012. Drugs. 2003. PMID: 12962524 Review.
-
[Sero-epidemiologic study of measles, rubella and mumps in the infantile population of Salamanca].An Esp Pediatr. 1992 Apr;36(4):293-7. An Esp Pediatr. 1992. PMID: 1605414 Review. Spanish.
Cited by
-
Measles Outbreak in a Rural Population in Bahar District, Hamadan Province, West of Iran in 2018.J Res Health Sci. 2020 Feb 27;20(1):e00470. doi: 10.34172/jrhs.2020.05. J Res Health Sci. 2020. PMID: 32814694 Free PMC article.
-
Key genes and pathways in measles and their interaction with environmental chemicals.Exp Ther Med. 2018 Jun;15(6):4890-4900. doi: 10.3892/etm.2018.6050. Epub 2018 Apr 11. Exp Ther Med. 2018. PMID: 29805511 Free PMC article.
-
Impact of the two-dose rubella vaccination regimen on incidence of rubella seronegativity in gravidae aged 25 years and younger.PLoS One. 2017 Aug 30;12(8):e0183630. doi: 10.1371/journal.pone.0183630. eCollection 2017. PLoS One. 2017. PMID: 28854204 Free PMC article.
-
Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population.Vaccines (Basel). 2025 Feb 27;13(3):249. doi: 10.3390/vaccines13030249. Vaccines (Basel). 2025. PMID: 40266124 Free PMC article.
-
Immunogenicity of mumps virus vaccine candidates matching circulating genotypes in the United States and China.Vaccine. 2017 Jul 13;35(32):3988-3994. doi: 10.1016/j.vaccine.2017.05.084. Epub 2017 Jun 13. Vaccine. 2017. PMID: 28623030 Free PMC article.
References
-
- Liu YB, Lu PS, Hu Y, Wang ZG, Deng XY, et al. (2013) Cross-Sectional Surveys of Measles Antibodies in the Jiangsu Province of China from 2008 to 2010: The effect of high coverage with two doses of measles vaccine among children. PLoS ONE 8(6): e66771 10.1371/journal.pone.0066771 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

