In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression
- PMID: 24586731
- PMCID: PMC3930725
- DOI: 10.1371/journal.pone.0089377
In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression
Abstract
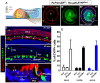
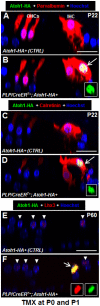
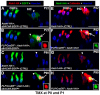
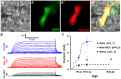
Regeneration of auditory hair cells (HCs) is a promising approach to restore hearing. Recent studies have demonstrated that induced pluripotent stem cells/embryonic stem cells or supporting cells (SCs) adjacent to HCs can be converted to adopt the HC fate. However, little is known about whether new HCs are characteristic of outer or inner HCs. Here, we showed in vivo conversion of 2 subtypes of SCs, inner border cells (IBs) and inner phalangeal cells (IPhs), to the inner HC (IHC) fate. This was achieved by ectopically activating Atoh1, a transcription factor necessary for HC fate, in IBs/IPhs at birth. Atoh1+ IBs/IPhs first turned on Pou4f3, another HC transcription factor, before expressing 8 HC markers. The conversion rate gradually increased from ∼ 2.4% at 1 week of age to ∼ 17.8% in adult. Interestingly, new HCs exhibited IHC characteristics such as straight line-shaped stereociliary bundles, expression of Fgf8 and otoferlin, and presence of larger outward currents than those of outer HCs. However, new HCs lacked the terminal differentiation IHC marker vGlut3, exhibited reduced density of presynaptic Cbtp2 puncta that had little postsynaptic GluR2 specialization, and displayed immature IHC outward currents. Our results demonstrate that the conversion rate of IBs/IPhs in vivo by Atoh1 ectopic expression into the IHC fate was higher and faster and the conversion was more complete than that of the 2 other SC subtypes underneath the outer HCs; however, these new IHCs are arrested before terminal differentiation. Thus, IBs/IPhs are good candidates to regenerate IHCs in vivo.
Conflict of interest statement
Figures






Similar articles
-
Generation of mature and functional hair cells by co-expression of Gfi1, Pou4f3, and Atoh1 in the postnatal mouse cochlea.Cell Rep. 2021 Apr 20;35(3):109016. doi: 10.1016/j.celrep.2021.109016. Cell Rep. 2021. PMID: 33882317
-
In Vivo Cochlear Hair Cell Generation and Survival by Coactivation of β-Catenin and Atoh1.J Neurosci. 2015 Jul 29;35(30):10786-98. doi: 10.1523/JNEUROSCI.0967-15.2015. J Neurosci. 2015. PMID: 26224861 Free PMC article.
-
In Vivo Interplay between p27Kip1, GATA3, ATOH1, and POU4F3 Converts Non-sensory Cells to Hair Cells in Adult Mice.Cell Rep. 2017 Apr 11;19(2):307-320. doi: 10.1016/j.celrep.2017.03.044. Cell Rep. 2017. PMID: 28402854 Free PMC article.
-
Atoh1 and other related key regulators in the development of auditory sensory epithelium in the mammalian inner ear: function and interplay.Dev Biol. 2019 Feb 15;446(2):133-141. doi: 10.1016/j.ydbio.2018.12.025. Epub 2018 Dec 31. Dev Biol. 2019. PMID: 30605626 Review.
-
Beyond generalized hair cells: molecular cues for hair cell types.Hear Res. 2013 Mar;297:30-41. doi: 10.1016/j.heares.2012.11.008. Epub 2012 Nov 27. Hear Res. 2013. PMID: 23201032 Free PMC article. Review.
Cited by
-
Three distinct Atoh1 enhancers cooperate for sound receptor hair cell development.Proc Natl Acad Sci U S A. 2022 Aug 9;119(32):e2119850119. doi: 10.1073/pnas.2119850119. Epub 2022 Aug 4. Proc Natl Acad Sci U S A. 2022. PMID: 35925886 Free PMC article.
-
Characterization of the transcriptomes of Atoh1-induced hair cells in the mouse cochlea.Am J Stem Cells. 2020 Feb 15;9(1):1-15. eCollection 2020. Am J Stem Cells. 2020. PMID: 32211215 Free PMC article.
-
Auditory hair cell-specific deletion of p27Kip1 in postnatal mice promotes cell-autonomous generation of new hair cells and normal hearing.J Neurosci. 2014 Nov 19;34(47):15751-63. doi: 10.1523/JNEUROSCI.3200-14.2014. J Neurosci. 2014. PMID: 25411503 Free PMC article.
-
Quantitative Analysis of Supporting Cell Subtype Labeling Among CreER Lines in the Neonatal Mouse Cochlea.J Assoc Res Otolaryngol. 2017 Apr;18(2):227-245. doi: 10.1007/s10162-016-0598-0. Epub 2016 Nov 21. J Assoc Res Otolaryngol. 2017. PMID: 27873085 Free PMC article.
-
Pseudo-immortalization of postnatal cochlear progenitor cells yields a scalable cell line capable of transcriptionally regulating mature hair cell genes.Sci Rep. 2015 Dec 7;5:17792. doi: 10.1038/srep17792. Sci Rep. 2015. PMID: 26639154 Free PMC article.
References
-
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, et al. (1999) Math1: an essential gene for the generation of inner ear hair cells. Science 284: 1837–1841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

