Comparison of the 'chemical' and 'structural' approaches to the optimization of the thrombin-binding aptamer
- PMID: 24586736
- PMCID: PMC3930721
- DOI: 10.1371/journal.pone.0089383
Comparison of the 'chemical' and 'structural' approaches to the optimization of the thrombin-binding aptamer
Abstract
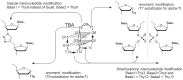
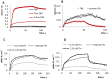
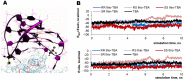
Noncanonically structured DNA aptamers to thrombin were examined. Two different approaches were used to improve stability, binding affinity and biological activity of a known thrombin-binding aptamer. These approaches are chemical modification and the addition of a duplex module to the aptamer core structure. Several chemically modified aptamers and the duplex-bearing ones were all studied under the same conditions by a set of widely known and some relatively new methods. A number of the thrombin-binding aptamer analogs have demonstrated improved characteristics. Most importantly, the study allowed us to compare directly the two approaches to aptamer optimization and to analyze their relative advantages and disadvantages as well as their potential in drug design and fundamental studies.
Conflict of interest statement
Figures
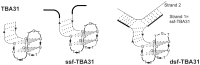
Similar articles
-
Synthesis, characterization and in vitro activity of thrombin-binding DNA aptamers with triazole internucleotide linkages.Eur J Med Chem. 2013 Sep;67:90-7. doi: 10.1016/j.ejmech.2013.06.034. Epub 2013 Jun 25. Eur J Med Chem. 2013. PMID: 23850569
-
Different duplex/quadruplex junctions determine the properties of anti-thrombin aptamers with mixed folding.Nucleic Acids Res. 2016 Jan 29;44(2):983-91. doi: 10.1093/nar/gkv1384. Epub 2015 Dec 15. Nucleic Acids Res. 2016. PMID: 26673709 Free PMC article.
-
Manipulation of a DNA aptamer-protein binding site through arylation of internal guanine residues.Org Biomol Chem. 2018 May 23;16(20):3831-3840. doi: 10.1039/c8ob00704g. Org Biomol Chem. 2018. PMID: 29745412
-
G-quadruplex-based aptamers targeting human thrombin: Discovery, chemical modifications and antithrombotic effects.Pharmacol Ther. 2021 Jan;217:107649. doi: 10.1016/j.pharmthera.2020.107649. Epub 2020 Aug 7. Pharmacol Ther. 2021. PMID: 32777331 Review.
-
Thrombin binding aptamer, more than a simple aptamer: chemically modified derivatives and biomedical applications.Curr Pharm Des. 2012;18(14):2036-47. doi: 10.2174/138161212799958387. Curr Pharm Des. 2012. PMID: 22376107 Review.
Cited by
-
Responses of DNA Mismatch Repair Proteins to a Stable G-Quadruplex Embedded into a DNA Duplex Structure.Int J Mol Sci. 2020 Nov 20;21(22):8773. doi: 10.3390/ijms21228773. Int J Mol Sci. 2020. PMID: 33233554 Free PMC article.
-
Trends in the Design and Development of Specific Aptamers Against Peptides and Proteins.Protein J. 2016 Apr;35(2):81-99. doi: 10.1007/s10930-016-9653-2. Protein J. 2016. PMID: 26984473 Review.
-
Aptamer-Functionalized Nanoparticles as "Smart Bombs": The Unrealized Potential for Personalized Medicine and Targeted Cancer Treatment.Target Oncol. 2015 Dec;10(4):467-85. doi: 10.1007/s11523-015-0371-z. Target Oncol. 2015. PMID: 25989948 Review.
-
Novel isoguanine derivative of unlocked nucleic acid-Investigations of thermodynamics and biological potential of modified thrombin binding aptamer.PLoS One. 2018 May 24;13(5):e0197835. doi: 10.1371/journal.pone.0197835. eCollection 2018. PLoS One. 2018. PMID: 29795635 Free PMC article.
-
Impact of G-Quadruplexes on the Regulation of Genome Integrity, DNA Damage and Repair.Biomolecules. 2021 Aug 27;11(9):1284. doi: 10.3390/biom11091284. Biomolecules. 2021. PMID: 34572497 Free PMC article. Review.
References
-
- Stoltenburg R, Reinemann C, Strehlitz B (2007) SELEX–a (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol Eng 24: 381–403. - PubMed
-
- Griffin LC, Tidmarsh GF, Bock LC, Toole JJ, Leung LL (1993) In vivo anticoagulant properties of a novel nucleotide-based thrombin inhibitor and demonstration of regional anticoagulation in extracorporeal circuits. Blood 81: 3271–3276. - PubMed
-
- Padmanabhan K, Padmanabhan KP, Ferrara JD, Sadler JE, Tulinsky A (1993) The structure of alpha-thrombin inhibited by a 15-mer single-stranded DNA aptamer. J Biol Chem 268: 17651–17654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

