HTLV-1 Tax oncoprotein inhibits the estrogen-induced-ER α-Mediated BRCA1 expression by interaction with CBP/p300 cofactors
- PMID: 24586743
- PMCID: PMC3931753
- DOI: 10.1371/journal.pone.0089390
HTLV-1 Tax oncoprotein inhibits the estrogen-induced-ER α-Mediated BRCA1 expression by interaction with CBP/p300 cofactors
Abstract
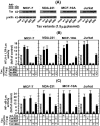
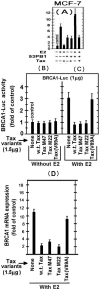
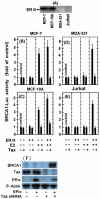
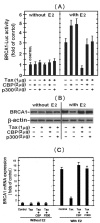
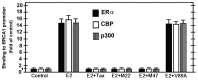
BRCA1 is a multifunctional tumor suppressor, whose expression is activated by the estrogen (E2)-liganded ERα receptor and regulated by certain recruited transcriptional co-activators. Interference with BRCA1 expression and/or functions leads to high risk of breast or/and ovarian cancer. Another multifunctional protein, HTLV-1Tax oncoprotein, is widely regarded as crucial for developing adult T-cell leukemia and other clinical disorders. Tax profile reveals that it can antagonize BRCA1 expression and/or functionality. Therefore, we hypothesize that Tax expression in breast cells can sensitize them to malignant transformation by environmental carcinogens. Here we examined Tax effect on BRCA1 expression by testing its influence on E2-induced expression of BRCA1 promoter-driven luciferase reporter (BRCA1-Luc). We found that E2 strongly stimulated this reporter expression by liganding to ERα, which consequently associated with BRCA1 promoter, while ERα concomitantly recruited CBP/p300 to this complex for co-operative enhancement of BRCA1 expression. Introducing Tax into these cells strongly blocked this E2-ERα-mediated activation of BRCA1 expression. We noted, also, that Tax exerted this inhibition by binding to CBP/p300 without releasing them from their complex with ERα. Chip assay revealed that the binding of Tax to the CBP/p300-ERα complex, prevented its link to AP1 site. Interestingly, we noted that elevating the intracellular pool of CBP or p300 to excessive levels dramatically reduced the Tax-mediated inhibition of BRCA1 expression. Exploring the mechanism of this reduction revealed that the excessive co-factors were sufficient to bind separately the free Tax molecules, thus lowering their amount in the CBP/p300-ERα complex and relieving, thereby, the inhibition of BRCA1 expression.
Conflict of interest statement
Figures

References
-
- Martin M (2006) Molecular biology of breast cancer. Clin Transl Oncol 8: 7–14. - PubMed
-
- Rosen EM, Fan S, Pestell RG, Goldberg ID (2003) BRCA1 gene in breast cancer. J Cell Physiol 196: 19–41. - PubMed
-
- Dumitrescu RG, Shields PG (2005) The etiology of alcohol-induced breast cancer. Alcohol 35: 213–225. - PubMed
-
- Rosen EM, Fan S, Ma Y (2006) BRCA1 regulation of transcription. Cancer Lett 236: 175–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

