Mutation or loss of p53 differentially modifies TGFβ action in ovarian cancer
- PMID: 24586866
- PMCID: PMC3930740
- DOI: 10.1371/journal.pone.0089553
Mutation or loss of p53 differentially modifies TGFβ action in ovarian cancer
Abstract
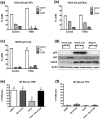
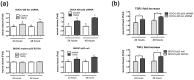
Ovarian cancer is the most lethal gynecological disease affecting women in the US. The Cancer Genome Atlas Network identified p53 mutations in 96% of high-grade serous ovarian carcinomas, demonstrating its critical role. Additionally, the Transforming Growth Factor Beta (TGFβ) pathway is dysfunctional in various malignancies, including ovarian cancer. This study investigated how expression of wild-type, mutant, or the absence of p53 alters ovarian cancer cell response to TGFβ signaling, as well as the response of the ovarian surface epithelium and the fallopian tube epithelium to TGFβ. Only ovarian cancer cells expressing wild-type p53 were growth inhibited by TGFβ, while ovarian cancer cells that were mutant or null p53 were not. TGFβ induced migration in p53 null SKOV3 cells, which was not observed in SKOV3 cells with stable expression of mutant p53 R273H. Knockdown of wild-type p53 in the OVCA 420 ovarian cancer cells enhanced cell migration in response to TGFβ. Increased protein expression of DKK1 and TMEPAI, two pro-invasive genes with enhanced expression in late stage metastatic ovarian cancer, was observed in p53 knockdown and null cells, while cells stably expressing mutant p53 demonstrated lower DKK1 and TMEPAI induction. Expression of mutant p53 or loss of p53 permit continued proliferation of ovarian cancer cell lines in the presence of TGFβ; however, cells expressing mutant p53 exhibit reduced migration and decreased protein levels of DKK1 and TMEPAI.
Conflict of interest statement
Figures



References
-
- Cordenonsi M, Dupont S, Maretto S, Insinga A, Imbriano C, et al. (2003) Links between tumor suppressors: p53 is required for TGF-beta gene responses by cooperating with Smads. Cell 113: 301–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

