Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes
- PMID: 24586872
- PMCID: PMC3938467
- DOI: 10.1371/journal.pone.0089561
Blockade of the programmed death-1 (PD1) pathway undermines potent genetic protection from type 1 diabetes
Abstract
Aims/hypothesis: Inhibition of PD1-PDL1 signaling in NOD mice accelerates onset of type 1 diabetes implicating this pathway in suppressing the emergence of pancreatic beta cell reactive T-cells. However, the molecular mechanism by which PD1 signaling protects from type 1 diabetes is not clear. We hypothesized that differential susceptibility of Idd mouse strains to type 1 diabetes when challenged with anti PDL1 will identify genomic loci that collaborate with PD1 signaling in suppressing type 1 diabetes.
Methods: Anti PDL1 was administered to NOD and various Idd mouse strains at 10 weeks of age and onset of disease was monitored by measuring blood glucose levels. Additionally, histological evaluation of the pancreas was performed to determine degree of insulitis. Statistical analysis of the data was performed using Log-Rank and Student's t-test.
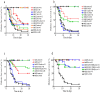
Results: Blockade of PDL1 rapidly precipitated type 1 diabetes in nearly all NOD Idd congenic strains tested, despite the fact that all are moderately (Idd5, Idd3 and Idd10/18) or highly (Idd3/10/18 and Idd9) protected from spontaneous type 1 diabetes by virtue of their protective Idd genes. Only the Idd3/5 strain, which is nearly 100% protected from spontaneous disease, remained normoglycemic following PDL1 blockade.
Conclusions: These results indicate that multiple Idd loci collaborate with PD1 signaling. Anti PDL1 treatment undermines a large portion of the genetic protection mediated by Idd genes in the NOD model of type 1 diabetes. Basal insulitis correlated with higher susceptibility to type 1 diabetes. These findings have important implications since the PD1 pathway is a target for immunotherapy.
Conflict of interest statement
Figures




References
-
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, et al. (2006) Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med 12: 1198–1202. - PubMed
-
- Guleria I, Gubbels Bupp M, Dada S, Fife B, Tang Q, et al. (2007) Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol 125: 16–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

