Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway
- PMID: 24586874
- PMCID: PMC3931808
- DOI: 10.1371/journal.pone.0089563
Local anesthetics induce apoptosis in human thyroid cancer cells through the mitogen-activated protein kinase pathway
Abstract
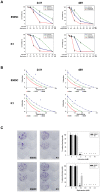
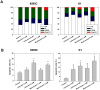
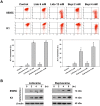
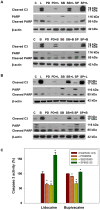
Local anesthetics are frequently used in fine-needle aspiration of thyroid lesions and locoregional control of persistent or recurrent thyroid cancer. Recent evidence suggests that local anesthetics have a broad spectrum of effects including inhibition of cell proliferation and induction of apoptosis in neuronal and other types of cells. In this study, we demonstrated that treatment with lidocaine and bupivacaine resulted in decreased cell viability and colony formation of both 8505C and K1 cells in a dose-dependent manner. Lidocaine and bupivacaine induced apoptosis, and necrosis in high concentrations, as determined by flow cytometry. Lidocaine and bupivacaine caused disruption of mitochondrial membrane potential and release of cytochrome c, accompanied by activation of caspase 3 and 7, PARP cleavage, and induction of a higher ratio of Bax/Bcl-2. Based on microarray and pathway analysis, apoptosis is the prominent transcriptional change common to lidocaine and bupivacaine treatment. Furthermore, lidocaine and bupivacaine attenuated extracellular signal-regulated kinase 1/2 (ERK1/2) activity and induced activation of p38 mitogen-activated protein kinase (MAPK) and c-jun N-terminal kinase. Pharmacological inhibitors of MAPK/ERK kinase and p38 MAPK suppressed caspase 3 activation and PARP cleavage. Taken together, our results for the first time demonstrate the cytotoxic effects of local anesthetics on thyroid cancer cells and implicate the MAPK pathways as an important mechanism. Our findings have potential clinical relevance in that the use of local anesthetics may confer previously unrecognized benefits in the management of patients with thyroid cancer.
Conflict of interest statement
Figures


References
-
- Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM (2010) Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery 148: 1147–1152. - PubMed
-
- Mazzaferri EL, Kloos RT (2001) Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 86: 1447–1463. - PubMed
-
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. (2009) American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19: 1167–1214. - PubMed
-
- Lewis BD, Hay ID, Charboneau JW, McIver B, Reading CC, et al. (2002) Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol 178: 699–704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

