Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval
- PMID: 24586903
- PMCID: PMC3931797
- DOI: 10.1371/journal.pone.0089599
Serum and glucocorticoid-inducible kinase1 increases plasma membrane wt-CFTR in human airway epithelial cells by inhibiting its endocytic retrieval
Abstract
Background: Chloride (Cl) secretion by the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) located in the apical membrane of respiratory epithelial cells plays a critical role in maintenance of the airway surface liquid and mucociliary clearance of pathogens. Previously, we and others have shown that the serum and glucocorticoid-inducible kinase-1 (SGK1) increases wild type CFTR (wt-CFTR) mediated Cl transport in Xenopus oocytes by increasing the amount of wt-CFTR protein in the plasma membrane. However, the effect of SGK1 on the membrane abundance of wt-CFTR in airway epithelial cells has not been examined, and the mechanism whereby SGK1 increases membrane wt-CFTR has also not been examined. Thus, the goal of this study was to elucidate the mechanism whereby SGK1 regulates the membrane abundance of wt-CFTR in human airway epithelial cells.
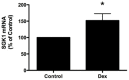
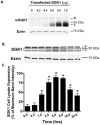
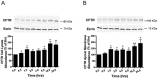
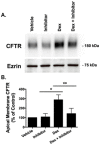
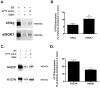
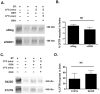
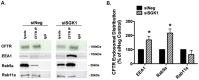
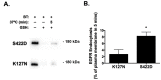
Methods and results: We report that elevated levels of SGK1, induced by dexamethasone, increase plasma membrane abundance of wt-CFTR. Reduction of SGK1 expression by siRNA (siSGK1) and inhibition of SGK1 activity by the SGK inhibitor GSK 650394 abrogated the ability of dexamethasone to increase plasma membrane wt-CFTR. Overexpression of a constitutively active SGK1 (SGK1-S422D) increased plasma membrane abundance of wt-CFTR. To understand the mechanism whereby SGK1 increased plasma membrane wt-CFTR, we examined the effects of siSGK1 and SGK1-S442D on the endocytic retrieval of wt-CFTR. While siSGK1 increased wt-CFTR endocytosis, SGK1-S442D inhibited CFTR endocytosis. Neither siSGK1 nor SGK1-S442D altered the recycling of endocytosed wt-CFTR back to the plasma membrane. By contrast, SGK1 increased the endocytosis of the epidermal growth factor receptor (EGFR).
Conclusion: This study demonstrates for the first time that SGK1 selectively increases wt-CFTR in the plasma membrane of human airway epithelia cells by inhibiting its endocytic retrieval from the membrane.
Conflict of interest statement
Figures

Similar articles
-
Serum- and glucocorticoid-induced protein kinase 1 (SGK1) increases the cystic fibrosis transmembrane conductance regulator (CFTR) in airway epithelial cells by phosphorylating Shank2E protein.J Biol Chem. 2014 Jun 13;289(24):17142-50. doi: 10.1074/jbc.M114.555599. Epub 2014 May 8. J Biol Chem. 2014. PMID: 24811177 Free PMC article.
-
Regulation of human cystic fibrosis transmembrane conductance regulator (CFTR) by serum- and glucocorticoid-inducible kinase (SGK1).Cell Physiol Biochem. 2007;20(1-4):91-8. doi: 10.1159/000104157. Cell Physiol Biochem. 2007. PMID: 17595519
-
The deubiquitinating enzyme USP10 regulates the endocytic recycling of CFTR in airway epithelial cells.Channels (Austin). 2010 May-Jun;4(3):150-4. doi: 10.4161/chan.4.3.11223. Channels (Austin). 2010. PMID: 20215869 Free PMC article.
-
CFTR, chloride concentration and cell volume: could mammalian protein histidine phosphorylation play a latent role?Exp Physiol. 2006 Jan;91(1):131-9. doi: 10.1113/expphysiol.2005.031823. Epub 2005 Oct 11. Exp Physiol. 2006. PMID: 16219660 Review.
-
CFTR-NHERF2-LPA₂ Complex in the Airway and Gut Epithelia.Int J Mol Sci. 2017 Sep 4;18(9):1896. doi: 10.3390/ijms18091896. Int J Mol Sci. 2017. PMID: 28869532 Free PMC article. Review.
Cited by
-
Signaling Cascade Involved in Rapid Stimulation of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) by Dexamethasone.Int J Mol Sci. 2017 Aug 19;18(8):1807. doi: 10.3390/ijms18081807. Int J Mol Sci. 2017. PMID: 28825630 Free PMC article.
-
Glucocorticoids Distinctively Modulate the CFTR Channel with Possible Implications in Lung Development and Transition into Extrauterine Life.PLoS One. 2015 Apr 24;10(4):e0124833. doi: 10.1371/journal.pone.0124833. eCollection 2015. PLoS One. 2015. PMID: 25910246 Free PMC article.
-
Effects of Pseudomonas aeruginosa on CFTR chloride secretion and the host immune response.Am J Physiol Cell Physiol. 2017 Apr 1;312(4):C357-C366. doi: 10.1152/ajpcell.00373.2016. Epub 2017 Jan 25. Am J Physiol Cell Physiol. 2017. PMID: 28122735 Free PMC article. Review.
-
Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID).J Clin Med. 2022 Jul 19;11(14):4179. doi: 10.3390/jcm11144179. J Clin Med. 2022. PMID: 35887942 Free PMC article.
-
Cystic fibrosis transmembrane conductance regulator in COPD: a role in respiratory epithelium and beyond.Eur Respir J. 2023 Apr 1;61(4):2201307. doi: 10.1183/13993003.01307-2022. Print 2023 Apr. Eur Respir J. 2023. PMID: 37003609 Free PMC article. Review.
References
-
- Guggino WB, Stanton BA (2006) New insights into cystic fibrosis: molecular switches that regulate CFTR. Nature reviews Molecular cell biology 7: 426–436. - PubMed
-
- Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, et al. (2012) Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 26: 533–545. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

