Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts (CAFs) is suppressed by omega-3 polyunsaturated fatty acids in vitro and in vivo
- PMID: 24586907
- PMCID: PMC3937340
- DOI: 10.1371/journal.pone.0089605
Matrix metalloproteinase (MMP)-9 in cancer-associated fibroblasts (CAFs) is suppressed by omega-3 polyunsaturated fatty acids in vitro and in vivo
Abstract
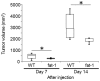
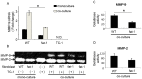
Cancer associated fibroblasts (CAFs) are responsible for tumor growth, angiogenesis, invasion, and metastasis. Matrix metalloproteinase (MMP)-9 secreted from cancer stroma populated by CAFs is a prerequisite for cancer angiogenesis and metastasis. Omega-3 polyunsaturated fatty acids (omega-3 PUFA) have been reported to have anti-tumor effects on diverse types of malignancies. Fat-1 mice, which can convert omega-6 to omega-3 PUFA independent of diet, are useful to investigate the functions of endogenous omega-3 PUFA. To examine the effect of omega-3 PUFA on tumorigenesis, TC-1 cells, a murine epithelial cell line immortalized by human papillomavirus (HPV) oncogenes, were injected subcutaneously into fat-1 or wild type mice. Tumor growth and angiogenesis of the TC-1 tumor were significantly suppressed in fat-1 compared to wild type mice. cDNA microarray of the tumors derived from fat-1 and wild type mice revealed that MMP-9 is downregulated in fat-1 mice. Immunohistochemical study demonstrated immunoreactivity for MMP-9 in the tumor stromal fibroblasts was diffusely positive in wild type whereas focal in fat-1 mice. MMP-9 was expressed in primary cultured fibroblasts isolated from fat-1 and wild type mice but was not expressed in TC-1 cells. Co-culture of fibroblasts with TC-1 cells enhanced the expression and the proteinase activity of MMP-9, although the protease activity of MMP-9 in fat-1-derived fibroblasts was lower than that in wild type fibroblasts. Our data suggests that omega-3 PUFAs suppress MMP-9 induction and tumor angiogenesis. These findings may provide insight into mechanisms by which omega-3 PUFAs exert anti-tumor effects by modulating tumor microenvironment.
Conflict of interest statement
Figures



Similar articles
-
Modulation of angiogenesis by omega-3 polyunsaturated fatty acids is mediated by cyclooxygenases.Blood. 2008 Apr 1;111(7):3514-21. doi: 10.1182/blood-2007-08-109934. Epub 2008 Jan 23. Blood. 2008. PMID: 18216296
-
Dietary supplementation with omega-3 polyunsaturated fatty acids modulate matrix metalloproteinase immunoreactivity in a mouse model of pre-abdominal aortic aneurysm.Heart Lung Circ. 2015 Apr;24(4):377-85. doi: 10.1016/j.hlc.2014.11.005. Epub 2014 Nov 20. Heart Lung Circ. 2015. PMID: 25512019
-
Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells--implications for complementary multiple sclerosis treatment.Neurochem Res. 2007 Dec;32(12):2184-93. doi: 10.1007/s11064-007-9415-9. Epub 2007 Jul 11. Neurochem Res. 2007. PMID: 17624613
-
Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells.Differentiation. 2002 Dec;70(9-10):486-97. doi: 10.1046/j.1432-0436.2002.700903.x. Differentiation. 2002. PMID: 12492491 Review.
-
ω-3 polyunsaturated fatty acids-derived lipid metabolites on angiogenesis, inflammation and cancer.Prostaglandins Other Lipid Mediat. 2014 Oct;113-115:13-20. doi: 10.1016/j.prostaglandins.2014.07.002. Epub 2014 Jul 11. Prostaglandins Other Lipid Mediat. 2014. PMID: 25019221 Free PMC article. Review.
Cited by
-
Revisiting Cancer Stem Cells as the Origin of Cancer-Associated Cells in the Tumor Microenvironment: A Hypothetical View from the Potential of iPSCs.Cancers (Basel). 2020 Apr 4;12(4):879. doi: 10.3390/cancers12040879. Cancers (Basel). 2020. PMID: 32260363 Free PMC article. Review.
-
Impact of high glucose on metastasis of colon cancer cells.World J Gastroenterol. 2015 Feb 21;21(7):2047-57. doi: 10.3748/wjg.v21.i7.2047. World J Gastroenterol. 2015. PMID: 25717237 Free PMC article.
-
Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth.Cancers (Basel). 2015 Dec 11;7(4):2443-58. doi: 10.3390/cancers7040902. Cancers (Basel). 2015. PMID: 26690480 Free PMC article. Review.
-
Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature.Matrix Biol. 2015 May-Jul;44-46:94-112. doi: 10.1016/j.matbio.2015.04.004. Epub 2015 Apr 22. Matrix Biol. 2015. PMID: 25912949 Free PMC article. Review.
-
High NOTCH1 mRNA Expression Is Associated with Better Survival in HNSCC.Int J Mol Sci. 2018 Mar 13;19(3):830. doi: 10.3390/ijms19030830. Int J Mol Sci. 2018. PMID: 29533972 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

