MAP3K8 (TPL2/COT) affects obesity-induced adipose tissue inflammation without systemic effects in humans and in mice
- PMID: 24586913
- PMCID: PMC3933658
- DOI: 10.1371/journal.pone.0089615
MAP3K8 (TPL2/COT) affects obesity-induced adipose tissue inflammation without systemic effects in humans and in mice
Abstract
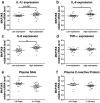
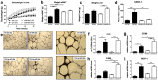
Chronic low-grade inflammation in adipose tissue often accompanies obesity, leading to insulin resistance and increasing the risk for metabolic diseases. MAP3K8 (TPL2/COT) is an important signal transductor and activator of pro-inflammatory pathways that has been linked to obesity-induced adipose tissue inflammation. We used human adipose tissue biopsies to study the relationship of MAP3K8 expression with markers of obesity and expression of pro-inflammatory cytokines (IL-1β, IL-6 and IL-8). Moreover, we evaluated obesity-induced adipose tissue inflammation and insulin resistance in mice lacking MAP3K8 and WT mice on a high-fat diet (HFD) for 16 weeks. Individuals with a BMI >30 displayed a higher mRNA expression of MAP3K8 in adipose tissue compared to individuals with a normal BMI. Additionally, high mRNA expression levels of IL-1β, IL-6 and IL-8, but not TNF -α, in human adipose tissue were associated with higher expression of MAP3K8. Moreover, high plasma SAA and CRP did not associate with increased MAP3K8 expression in adipose tissue. Similarly, no association was found for MAP3K8 expression with plasma insulin or glucose levels. Mice lacking MAP3K8 had similar bodyweight gain as WT mice, yet displayed lower mRNA expression levels of IL-1β, IL-6 and CXCL1 in adipose tissue in response to the HFD as compared to WT animals. However, MAP3K8 deficient mice were not protected against HFD-induced adipose tissue macrophage infiltration or the development of insulin resistance. Together, the data in both human and mouse show that MAP3K8 is involved in local adipose tissue inflammation, specifically for IL-1β and its responsive cytokines IL-6 and IL-8, but does not seem to have systemic effects on insulin resistance.
Conflict of interest statement
Figures



Similar articles
-
Tpl2 kinase is upregulated in adipose tissue in obesity and may mediate interleukin-1beta and tumor necrosis factor-{alpha} effects on extracellular signal-regulated kinase activation and lipolysis.Diabetes. 2010 Jan;59(1):61-70. doi: 10.2337/db09-0470. Epub 2009 Oct 6. Diabetes. 2010. PMID: 19808894 Free PMC article.
-
Tumor progression locus 2 (TPL2) regulates obesity-associated inflammation and insulin resistance.Diabetes. 2011 Apr;60(4):1168-76. doi: 10.2337/db10-0715. Epub 2011 Feb 23. Diabetes. 2011. PMID: 21346175 Free PMC article.
-
Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis.Diabetes. 2011 Jun;60(6):1688-98. doi: 10.2337/db10-1278. Epub 2011 Apr 22. Diabetes. 2011. PMID: 21515850 Free PMC article.
-
Recent advances in the relationship between obesity, inflammation, and insulin resistance.Eur Cytokine Netw. 2006 Mar;17(1):4-12. Eur Cytokine Netw. 2006. PMID: 16613757 Review.
-
Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance.Biochim Biophys Acta. 2014 Mar;1842(3):446-62. doi: 10.1016/j.bbadis.2013.05.017. Epub 2013 May 22. Biochim Biophys Acta. 2014. PMID: 23707515 Free PMC article. Review.
Cited by
-
Novel role of circRSU1 in the progression of osteoarthritis by adjusting oxidative stress.Theranostics. 2021 Jan 1;11(4):1877-1900. doi: 10.7150/thno.53307. eCollection 2021. Theranostics. 2021. PMID: 33408787 Free PMC article.
-
Transcriptomic analysis of LMH cells in response to the overexpression of a protein of Eimeria tenella encoded by the locus ETH_00028350.Front Vet Sci. 2022 Nov 21;9:1053701. doi: 10.3389/fvets.2022.1053701. eCollection 2022. Front Vet Sci. 2022. PMID: 36478946 Free PMC article.
-
Aberrant expression of COT is related to recurrence of papillary thyroid cancer.Medicine (Baltimore). 2015 Feb;94(6):e548. doi: 10.1097/MD.0000000000000548. Medicine (Baltimore). 2015. PMID: 25674762 Free PMC article.
-
Alanine Aminotransferase and Body Composition in Obese Men and Women.Dis Markers. 2019 Aug 26;2019:1695874. doi: 10.1155/2019/1695874. eCollection 2019. Dis Markers. 2019. PMID: 31534560 Free PMC article.
-
Dysregulated expression of microRNAs and mRNAs in pulmonary artery remodeling in ascites syndrome in broiler chickens.Oncotarget. 2017 Jan 10;8(2):1993-2007. doi: 10.18632/oncotarget.12888. Oncotarget. 2017. PMID: 27791988 Free PMC article.
References
-
- Gregor MF, Hotamisligil GS (2011) Inflammatory Mechanisms in Obesity. Annu Rev Immunol. - PubMed
-
- Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11: 98–107. - PubMed
-
- Olefsky JM, Glass CK (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72: 219–246. - PubMed
-
- Tanti JF, Jager J (2009) Cellular mechanisms of insulin resistance: role of stress-regulated serine kinases and insulin receptor substrates (IRS) serine phosphorylation. Curr Opin Pharmacol 9: 753–762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

