Modulation by K+ Plus NH4+ of microsomal (Na+, K+)-ATPase activity in selected ontogenetic stages of the diadromous river shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae)
- PMID: 24586919
- PMCID: PMC3931822
- DOI: 10.1371/journal.pone.0089625
Modulation by K+ Plus NH4+ of microsomal (Na+, K+)-ATPase activity in selected ontogenetic stages of the diadromous river shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae)
Abstract
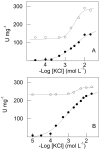
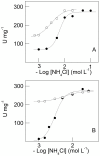
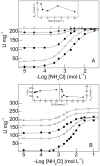
We investigate the synergistic stimulation by K(+) plus NH4 (+) of (Na(+), K(+))-ATPase activity in microsomal preparations of whole zoea I and decapodid III, and in juvenile and adult river shrimp gills. Modulation of (Na(+), K(+))-ATPase activity is ontogenetic stage-specific, and particularly distinct between juveniles and adults. Although both gill enzymes exhibit two different sites for K(+) and NH4 (+) binding, in the juvenile enzyme, these two sites are equivalent: binding by both ions results in slightly stimulated activity compared to that of a single ionic species. In the adult enzyme, the sites are not equivalent: when one ion occupies its specific binding site, (Na(+), K(+))-ATPase activity is stimulated synergistically by ≈ 50% on binding of the complementary ion. Immunolocalization reveals the enzyme to be distributed predominantly throughout the intralamellar septum in the gill lamellae of juveniles and adults. Western blot analyses demonstrate a single immunoreactive band, suggesting a single (Na(+), K(+))-ATPase α-subunit isoform that is distributed into different density membrane fractions, independently of ontogenetic stage. We propose a model for the modulation by K(+) and NH4 (+) of gill (Na(+), K(+))-ATPase activity. These findings suggest that the gill enzyme may be regulated by NH4 (+) during ontogenetic development in M. amazonicum.
Conflict of interest statement
Figures




Similar articles
-
A kinetic characterization of (Na+, K+)-ATPase activity in the gills of the pelagic seabob shrimp Xiphopenaeus kroyeri (Decapoda, Penaeidae).J Membr Biol. 2015 Apr;248(2):257-72. doi: 10.1007/s00232-014-9765-6. Epub 2014 Dec 23. J Membr Biol. 2015. PMID: 25534346
-
Kinetic characterization of the gill (Na+, K+)-ATPase in a hololimnetic population of the diadromous Amazon River shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae).Comp Biochem Physiol B Biochem Mol Biol. 2019 Jan;227:64-74. doi: 10.1016/j.cbpb.2018.09.004. Epub 2018 Sep 27. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 30267892
-
Kinetic analysis of gill (Na⁺,K⁺)-ATPase activity in selected ontogenetic stages of the Amazon River shrimp, Macrobrachium amazonicum (Decapoda, Palaemonidae): interactions at ATP- and cation-binding sites.J Membr Biol. 2012 Apr;245(4):201-15. doi: 10.1007/s00232-012-9431-9. Epub 2012 Apr 28. J Membr Biol. 2012. PMID: 22544049
-
Effects of ammonia stress in the Amazon river shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae).Aquat Toxicol. 2016 Jan;170:13-23. doi: 10.1016/j.aquatox.2015.10.021. Epub 2015 Nov 2. Aquat Toxicol. 2016. PMID: 26571214
-
Effects of ammonia on gill (Na+, K+)-ATPase kinetics in a hololimnetic population of the Amazon River shrimp Macrobrachium amazonicum.Aquat Toxicol. 2022 May;246:106144. doi: 10.1016/j.aquatox.2022.106144. Epub 2022 Mar 14. Aquat Toxicol. 2022. PMID: 35339850
Cited by
-
A kinetic characterization of (Na+, K+)-ATPase activity in the gills of the pelagic seabob shrimp Xiphopenaeus kroyeri (Decapoda, Penaeidae).J Membr Biol. 2015 Apr;248(2):257-72. doi: 10.1007/s00232-014-9765-6. Epub 2014 Dec 23. J Membr Biol. 2015. PMID: 25534346
-
Polyamines regulate phosphorylation-dephosphorylation kinetics in a crustacean gill (Na+, K+)-ATPase.Mol Cell Biochem. 2017 May;429(1-2):187-198. doi: 10.1007/s11010-017-2946-8. Epub 2017 Feb 11. Mol Cell Biochem. 2017. PMID: 28190171
-
A Kinetic Characterization of the Gill (Na+, K+)-ATPase from the Semi-terrestrial Mangrove Crab Cardisoma guanhumi Latreille, 1825 (Decapoda, Brachyura).J Membr Biol. 2017 Oct;250(5):517-534. doi: 10.1007/s00232-017-9978-6. Epub 2017 Aug 24. J Membr Biol. 2017. PMID: 28840273
-
Mechanisms of Na+ uptake from freshwater habitats in animals.Front Physiol. 2022 Oct 18;13:1006113. doi: 10.3389/fphys.2022.1006113. eCollection 2022. Front Physiol. 2022. PMID: 36388090 Free PMC article. Review.
-
Perfused Gills Reveal Fundamental Principles of pH Regulation and Ammonia Homeostasis in the Cephalopod Octopus vulgaris.Front Physiol. 2017 Mar 20;8:162. doi: 10.3389/fphys.2017.00162. eCollection 2017. Front Physiol. 2017. PMID: 28373845 Free PMC article.
References
-
- Jorgensen PL, Hakansson KO, Karlish SJD (2003) Structure and mechanism of Na,K-ATPase: Functional sites and their interactions. Ann Rev Physiol 65: 817–849. - PubMed
-
- Martin DW (2005) Structure-function relationships in the Na+, K+-pump. Semin Nephrol 25: 282–291. - PubMed
-
- Sáez AG, Lozano E, Zaldívar-Riverón A (2009) Evolutionary history of Na,K-ATPases and their osmoregulatory role. Genetica 136: 479–490. - PubMed
-
- Axelsen MG, Palmgren KB (1998) Evolution of P-type ATPases. Biochim Biophys Acta 1365: 37–45. - PubMed
-
- Jorgensen PL, Pedersen PA (2001) Structure-function relationships of Na+, K+, ATP, or Mg2+ binding and energy transduction in Na+,K+-ATPase. Biochim Biophys Acta 1505: 57–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

