Aeromonas hydrophila flagella glycosylation: involvement of a lipid carrier
- PMID: 24586923
- PMCID: PMC3931799
- DOI: 10.1371/journal.pone.0089630
Aeromonas hydrophila flagella glycosylation: involvement of a lipid carrier
Retraction in
-
Retraction: Aeromonas hydrophila flagella glycosylation: Involvement of a lipid carrier.PLoS One. 2025 May 9;20(5):e0324165. doi: 10.1371/journal.pone.0324165. eCollection 2025. PLoS One. 2025. PMID: 40343921 Free PMC article. No abstract available.
Abstract
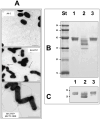
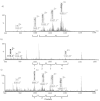
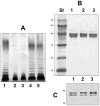
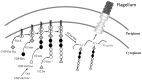
Polar flagellin proteins from Aeromonas hydrophila strain AH-3 (serotype O34) were found to be O-glycosylated with a heterogeneous glycan. Mutants unable to produce WecP or Gne enzymes showed altered motility, and the study of their polar flagellin glycosylation showed that the patterns of glycosylation differed from that observed with wild type polar flagellin. This suggested the involvement of a lipid carrier in glycosylation. A gene coding for an enzyme linking sugar to a lipid carrier was identified in strain AH-3 (WecX) and subsequent mutation abolished completely motility, flagella production by EM, and flagellin glycosylation. This is the first report of a lipid carrier involved in flagella O-glycosylation. A molecular model has been proposed. The results obtained suggested that the N-acetylhexosamines are N-acetylgalactosamines and that the heptasaccharide is completely independent of the O34-antigen lipopolysaccharide. Furthermore, by comparing the mutants with differing degrees of polar flagellin glycosylation, we established their importance in A. hydrophila flagella formation and motility.
© 2014 Merino et al. Except for Figure 7A panel 3, this is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

