Ectopic TLX1 expression accelerates malignancies in mice deficient in DNA-PK
- PMID: 24586935
- PMCID: PMC3935916
- DOI: 10.1371/journal.pone.0089649
Ectopic TLX1 expression accelerates malignancies in mice deficient in DNA-PK
Abstract
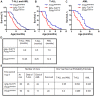
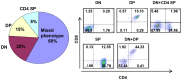
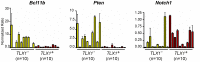
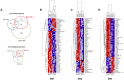
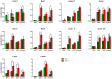
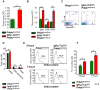
The noncluster homeobox gene HOX11/TLX1 (TLX1) is detected at the breakpoint of the t(10;14)(q24;q11) chromosome translocation in patients with T cell acute lymphoblastic leukemia (T-ALL). This translocation results in the inappropriate expression of TLX1 in T cells. The oncogenic potential of TLX1 was demonstrated in IgHμ-TLX1(Tg) mice which develop mature B cell lymphoma after a long latency period, suggesting the requirement of additional mutations to initiate malignancy. To determine whether dysregulation of genes involved in the DNA damage response contributed to tumor progression, we crossed IgHμ-TLX1(Tg) mice with mice deficient in the DNA repair enzyme DNA-PK (Prkdc(Scid/Scid) mice). IgHµ-TLX1(Tg)Prkdc(Scid/Scid) mice developed T-ALL and acute myeloid leukemia (AML) with reduced latency relative to control Prkdc(Scid/Scid) mice. Further analysis of thymi from premalignant mice revealed greater thymic cellularity concomitant with increased thymocyte proliferation and decreased apoptotic index. Moreover, premalignant and malignant thymocytes exhibited impaired spindle checkpoint function, in association with aneuploid karyotypes. Gene expression profiling of premalignant IgHµ-TLX1(Tg)Prkdc(Scid/Scid) thymocytes revealed dysregulated expression of cell cycle, apoptotic and mitotic spindle checkpoint genes in double negative 2 (DN2) and DN3 stage thymocytes. Collectively, these findings reveal a novel synergy between TLX1 and impaired DNA repair pathway in leukemogenesis.
Conflict of interest statement
Figures





References
-
- Dube ID, Kamel-Reid S, Yuan CC, Lu M, Wu X, et al. (1991) A novel human homeobox gene lies at the chromosome 10 breakpoint in lymphoid neoplasias with chromosomal translocation t(10;14). Blood 78: 2996–3003. - PubMed
-
- Hatano M, Roberts CW, Minden M, Crist WM, Korsmeyer SJ (1991) Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science 253: 79–82. - PubMed
-
- Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, et al. (2002) Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 1: 75–87. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

