Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage
- PMID: 24587004
- PMCID: PMC3931818
- DOI: 10.1371/journal.pone.0089743
Microvesicle shedding and lysosomal repair fulfill divergent cellular needs during the repair of streptolysin O-induced plasmalemmal damage
Abstract
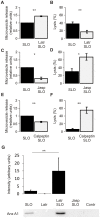
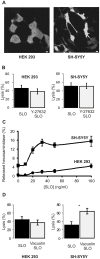
Pathogenic bacteria secrete pore-forming toxins that permeabilize the plasma membrane of host cells. Nucleated cells possess protective mechanisms that repair toxin-damaged plasmalemma. Currently two putative repair scenarios are debated: either the isolation of the damaged membrane regions and their subsequent expulsion as microvesicles (shedding) or lysosome-dependent repair might allow the cell to rid itself of its toxic cargo and prevent lysis. Here we provide evidence that both mechanisms operate in tandem but fulfill diverse cellular needs. The prevalence of the repair strategy varies between cell types and is guided by the severity and the localization of the initial toxin-induced damage, by the morphology of a cell and, most important, by the incidence of the secondary mechanical damage. The surgically precise action of microvesicle shedding is best suited for the instant elimination of individual toxin pores, whereas lysosomal repair is indispensable for mending of self-inflicted mechanical injuries following initial plasmalemmal permeabilization by bacterial toxins. Our study provides new insights into the functioning of non-immune cellular defenses against bacterial pathogens.
Conflict of interest statement
Figures






Similar articles
-
Down-regulation of acid sphingomyelinase and neutral sphingomyelinase-2 inversely determines the cellular resistance to plasmalemmal injury by pore-forming toxins.FASEB J. 2019 Jan;33(1):275-285. doi: 10.1096/fj.201800033R. Epub 2018 Jul 6. FASEB J. 2019. PMID: 29979630
-
Intrinsic repair protects cells from pore-forming toxins by microvesicle shedding.Cell Death Differ. 2017 May;24(5):798-808. doi: 10.1038/cdd.2017.11. Epub 2017 Feb 10. Cell Death Differ. 2017. PMID: 28186501 Free PMC article.
-
Active release of pneumolysin prepores and pores by mammalian cells undergoing a Streptococcus pneumoniae attack.Biochim Biophys Acta. 2016 Nov;1860(11 Pt A):2498-2509. doi: 10.1016/j.bbagen.2016.07.022. Epub 2016 Jul 30. Biochim Biophys Acta. 2016. PMID: 27481675
-
Defying death: Cellular survival strategies following plasmalemmal injury by bacterial toxins.Semin Cell Dev Biol. 2015 Sep;45:39-47. doi: 10.1016/j.semcdb.2015.10.016. Epub 2015 Oct 19. Semin Cell Dev Biol. 2015. PMID: 26481974 Review.
-
Plasma membrane repair and cellular damage control: the annexin survival kit.Biochem Pharmacol. 2011 Mar 15;81(6):703-12. doi: 10.1016/j.bcp.2010.12.027. Epub 2011 Jan 8. Biochem Pharmacol. 2011. PMID: 21219882 Review.
Cited by
-
Membrane Repair Mechanisms against Permeabilization by Pore-Forming Toxins.Toxins (Basel). 2018 Jun 9;10(6):234. doi: 10.3390/toxins10060234. Toxins (Basel). 2018. PMID: 29890730 Free PMC article. Review.
-
Chloramidine/Bisindolylmaleimide-I-Mediated Inhibition of Exosome and Microvesicle Release and Enhanced Efficacy of Cancer Chemotherapy.Int J Mol Sci. 2017 May 9;18(5):1007. doi: 10.3390/ijms18051007. Int J Mol Sci. 2017. PMID: 28486412 Free PMC article.
-
Muscle Cells Fix Breaches by Orchestrating a Membrane Repair Ballet.J Neuromuscul Dis. 2018;5(1):21-28. doi: 10.3233/JND-170251. J Neuromuscul Dis. 2018. PMID: 29480214 Free PMC article. Review.
-
Repair of a Bacterial Small β-Barrel Toxin Pore Depends on Channel Width.mBio. 2017 Feb 14;8(1):e02083-16. doi: 10.1128/mBio.02083-16. mBio. 2017. PMID: 28196960 Free PMC article.
-
Above the fray: Surface remodeling by secreted lysosomal enzymes leads to endocytosis-mediated plasma membrane repair.Semin Cell Dev Biol. 2015 Sep;45:10-7. doi: 10.1016/j.semcdb.2015.09.022. Epub 2015 Oct 1. Semin Cell Dev Biol. 2015. PMID: 26433178 Free PMC article. Review.
References
-
- Parker MW, Feil SC (2005) Pore-forming protein toxins: from structure to function. Prog Biophys Mol Biol 88: 91–142. - PubMed
-
- Draeger A, Monastyrskaya K, Babiychuk EB (2011) Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem Pharmacol 81: 703–712. - PubMed
-
- McNeil PL, Kirchhausen T (2005) An emergency response team for membrane repair. Nat Rev Mol Cell Biol 6: 499–505. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

