Evidence for a Golgi-to-endosome protein sorting pathway in Plasmodium falciparum
- PMID: 24587025
- PMCID: PMC3934947
- DOI: 10.1371/journal.pone.0089771
Evidence for a Golgi-to-endosome protein sorting pathway in Plasmodium falciparum
Abstract
During the asexual intraerythrocytic stage, the malaria parasite Plasmodium falciparum must traffic newly-synthesized proteins to a broad array of destinations within and beyond the parasite's plasma membrane. In this study, we have localized two well-conserved protein components of eukaryotic endosomes, the retromer complex and the small GTPase Rab7, to define a previously-undescribed endosomal compartment in P. falciparum. Retromer and Rab7 co-localized to a small number of punctate structures within parasites. These structures, which we refer to as endosomes, lie in close proximity to the Golgi apparatus and, like the Golgi apparatus, are inherited by daughter merozoites. However, the endosome is clearly distinct from the Golgi apparatus as neither retromer nor Rab7 redistributed to the endoplasmic reticulum upon brefeldin A treatment. Nascent rhoptries (specialized secretory organelles required for invasion) developed adjacent to endosomes, an observation that suggests a role for the endosome in rhoptry biogenesis. A P. falciparum homolog of the sortilin family of protein sorting receptors (PfSortilin) was localized to the Golgi apparatus. Together, these results elaborate a putative Golgi-to-endosome protein sorting pathway in asexual blood stage parasites and suggest that one role of retromer is to mediate the retrograde transport of PfSortilin from the endosome to the Golgi apparatus.
Conflict of interest statement
Figures
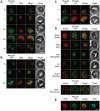


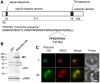
Similar articles
-
Retromer guides STxB and CD8-M6PR from early to recycling endosomes, EHD1 guides STxB from recycling endosome to Golgi.Traffic. 2012 Aug;13(8):1140-59. doi: 10.1111/j.1600-0854.2012.01374.x. Epub 2012 May 23. Traffic. 2012. PMID: 22540229 Free PMC article.
-
Evidence for Golgi-independent transport from the early secretory pathway to the plastid in malaria parasites.Mol Microbiol. 2006 Aug;61(3):614-30. doi: 10.1111/j.1365-2958.2006.05244.x. Epub 2006 Jun 20. Mol Microbiol. 2006. PMID: 16787449
-
SNX-BAR-mediated endosome tubulation is co-ordinated with endosome maturation.Traffic. 2012 Jan;13(1):94-107. doi: 10.1111/j.1600-0854.2011.01297.x. Epub 2011 Oct 31. Traffic. 2012. PMID: 21973056
-
Endosomal receptor trafficking: Retromer and beyond.Traffic. 2018 Aug;19(8):578-590. doi: 10.1111/tra.12574. Epub 2018 May 21. Traffic. 2018. PMID: 29667289 Free PMC article. Review.
-
Coats of endosomal protein sorting: retromer and ESCRT.Curr Opin Plant Biol. 2009 Dec;12(6):670-6. doi: 10.1016/j.pbi.2009.09.005. Epub 2009 Oct 15. Curr Opin Plant Biol. 2009. PMID: 19836992 Review.
Cited by
-
The Plasmodium falciparum homolog of Vps16 interacts with the core members of the Vps-C tethering complex.mSphere. 2025 Jul 29;10(7):e0028725. doi: 10.1128/msphere.00287-25. Epub 2025 Jul 8. mSphere. 2025. PMID: 40626728 Free PMC article.
-
Endosomal traffic disorders: a driving force behind neurodegenerative diseases.Transl Neurodegener. 2024 Dec 24;13(1):66. doi: 10.1186/s40035-024-00460-7. Transl Neurodegener. 2024. PMID: 39716330 Free PMC article. Review.
-
Resolving the homology-function relationship through comparative genomics of membrane-trafficking machinery and parasite cell biology.Mol Biochem Parasitol. 2016 Sep-Oct;209(1-2):88-103. doi: 10.1016/j.molbiopara.2016.07.003. Epub 2016 Jul 19. Mol Biochem Parasitol. 2016. PMID: 27444378 Free PMC article. Review.
-
Evidence that the Plasmodium falciparum Protein Sortilin Potentially Acts as an Escorter for the Trafficking of the Rhoptry-Associated Membrane Antigen to the Rhoptries.mSphere. 2018 Jan 3;3(1):e00551-17. doi: 10.1128/mSphere.00551-17. eCollection 2018 Jan-Feb. mSphere. 2018. PMID: 29299530 Free PMC article.
-
APEX2-based proximity proteomic analysis identifies candidate interactors for Plasmodium falciparum knob-associated histidine-rich protein in infected erythrocytes.Sci Rep. 2024 May 16;14(1):11242. doi: 10.1038/s41598-024-61295-w. Sci Rep. 2024. PMID: 38755230 Free PMC article.
References
-
- Wunderlich J, Rohrbach P, Dalton JP (2012) The malaria digestive vacuole. Front Biosci 4: 1424–1448. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

