Hypoxia exerts dualistic effects on inflammatory and proliferative responses of healthy and asthmatic primary human bronchial smooth muscle cells
- PMID: 24587090
- PMCID: PMC3933675
- DOI: 10.1371/journal.pone.0089875
Hypoxia exerts dualistic effects on inflammatory and proliferative responses of healthy and asthmatic primary human bronchial smooth muscle cells
Abstract
Background: For oxygen supply, airway wall cells depend on diffusion though the basement membrane, as well as on delivery by micro-vessels. In the asthmatic lung, local hypoxic conditions may occur due to increased thickness and altered composition of the basement membrane, as well as due to edema of the inflamed airway wall.
Objective: In our study we investigated the effect of hypoxia on proliferation and pro-inflammatory and pro-angiogenic parameter production by human bronchial smooth muscle cells (BSMC). Furthermore, conditioned media of hypoxia-exposed BSMC was tested for its ability to induce sprout outgrowth from endothelial cells spheroids.
Methods: BSMC were cultured in RPMI1640 (5% FCS) under normoxic (21% O₂) and hypoxic (1% and 5% O₂) conditions. Proliferation was determined by cell count and Western blot analysis for cyclin E and Proliferating Cell Nuclear Antigen (PCNA). Secretion of IL-6, IL-8, ENA-78 and VEGF-A was analyzed by ELISA. BSMC conditioned medium was tested for its angiogenic capacity by endothelial cell (EC)-spheroid in vitro angiogenesis assay.
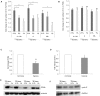
Results: Proliferation of BSMC obtained from asthmatic and non-asthmatic patients was significantly reduced in the presence of 1% O₂, whereas 5% O₂ reduced proliferation of asthmatic BSMC only. Hypoxia induced HIF-1α expression in asthmatic and non-asthmatic BSMC, which coincided with significantly increased release of IL-6, IL-8 and VEGF-A, but not ENA-78. Finally, endothelial sprout outgrowth from EC spheroids was increased when exposed to hypoxia conditioned BSMC medium.
Conclusion: Hypoxia had dualistic effects on proliferative and inflammatory responses of asthmatic and non-asthmatic BSMC. First, hypoxia reduced BSMC proliferation. Second, hypoxia induced a pro-inflammatory, pro-angiogenic response. BSMC and EC may thus be promising new targets to counteract and/or alleviate airway wall remodeling.
Conflict of interest statement
Figures




Similar articles
-
Bronchial smooth muscle cells of asthmatics promote angiogenesis through elevated secretion of CXC-chemokines (ENA-78, GRO-α, and IL-8).PLoS One. 2013 Dec 5;8(12):e81494. doi: 10.1371/journal.pone.0081494. eCollection 2013. PLoS One. 2013. PMID: 24339939 Free PMC article.
-
Bronchial epithelium-derived IL-8 and RANTES increased bronchial smooth muscle cell migration and proliferation by Krüppel-like factor 5 in areca nut-mediated airway remodeling.Toxicol Sci. 2011 May;121(1):177-90. doi: 10.1093/toxsci/kfr030. Epub 2011 Feb 4. Toxicol Sci. 2011. PMID: 21297082
-
Rhinoviral stimuli, epithelial factors and ATP signalling contribute to bronchial smooth muscle production of IL-33.J Transl Med. 2015 Aug 29;13:281. doi: 10.1186/s12967-015-0645-3. J Transl Med. 2015. PMID: 26318341 Free PMC article.
-
Involvement of fibronectin and matrix metalloproteinases in airway smooth muscle cell migration for the process of airway remodeling.Allergol Int. 2010 Sep;59(3):267-275. doi: 10.2332/allergolint.09-OA-0153. Epub 2010 May 25. Allergol Int. 2010. PMID: 20495339
-
Fibroblast growth factor 2 and transforming growth factor beta1 synergism in human bronchial smooth muscle cell proliferation.Am J Respir Cell Mol Biol. 2006 Jun;34(6):746-53. doi: 10.1165/rcmb.2005-0309OC. Epub 2006 Jan 26. Am J Respir Cell Mol Biol. 2006. PMID: 16439802
Cited by
-
17β-Estradiol Promotes Apoptosis in Airway Smooth Muscle Cells Through CD38/SIRT1/p53 Pathway.Front Endocrinol (Lausanne). 2018 Dec 19;9:770. doi: 10.3389/fendo.2018.00770. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30619097 Free PMC article.
-
Impact of Conditional miRNA126 Overexpression on Apoptosis-Resistant Endothelial Cell Production.PLoS One. 2015 May 11;10(5):e0126661. doi: 10.1371/journal.pone.0126661. eCollection 2015. PLoS One. 2015. PMID: 25961846 Free PMC article.
-
Dual effects of leptin in perioperative gas exchange of morbidly obese patients.PLoS One. 2018 Jul 5;13(7):e0199610. doi: 10.1371/journal.pone.0199610. eCollection 2018. PLoS One. 2018. PMID: 29975721 Free PMC article.
-
Perinatal oxygen in the developing lung.Can J Physiol Pharmacol. 2015 Feb;93(2):119-27. doi: 10.1139/cjpp-2014-0387. Epub 2014 Dec 9. Can J Physiol Pharmacol. 2015. PMID: 25594569 Free PMC article. Review.
-
The Three A's in Asthma - Airway Smooth Muscle, Airway Remodeling & Angiogenesis.Open Respir Med J. 2015 Jun 17;9:70-80. doi: 10.2174/1874306401509010070. eCollection 2015. Open Respir Med J. 2015. PMID: 26106455 Free PMC article.
References
-
- Johnson PR, Roth M, Tamm M, Hughes M, Ge Q, et al. Airway smooth muscle cell proliferation is increased in asthma. American journal of respiratory and critical care medicine. 164(3): 474–7. - PubMed
-
- Borger P, Matsumoto H, Boustany S, Gencay MM, Burgess JK, et al. Disease-specific expression and regulation of CCAAT/enhancer-binding proteins in asthma and chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 119(1): 98–105. - PubMed
-
- Borger P, Miglino N, Baraket M, Black JL, Tamm M, et al. Impaired translation of CCAAT/enhancer binding protein alpha mRNA in bronchial smooth muscle cells of asthmatic patients. The Journal of allergy and clinical immunology. 123(3): 639–45. - PubMed
-
- Miglino N, Roth M, Tamm M, Borger P. House dust mite extract downregulates C/EBPalpha in asthmatic bronchial smooth muscle cells. The European respiratory journal : official journal of the European Society for Clinical Respiratory Physiology. 38(1): 50–8. - PubMed
-
- Roth M, Johnson PR, Borger P, Bihl MP, Rüdiger JJ, et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. The New England journal of medicine. 351(6): 560–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

