Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells
- PMID: 24587097
- PMCID: PMC3931832
- DOI: 10.1371/journal.pone.0089882
Pharmacokinetics of natural and engineered secreted factors delivered by mesenchymal stromal cells
Erratum in
- PLoS One. 2014;9(6):e99813. Murray, Ryan M [corrected to Murray, Ryan C]
Abstract
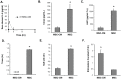
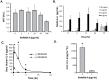
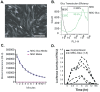
Transient cell therapy is an emerging drug class that requires new approaches for pharmacological monitoring during use. Human mesenchymal stem cells (MSCs) are a clinically-tested transient cell therapeutic that naturally secrete anti-inflammatory factors to attenuate immune-mediated diseases. MSCs were used as a proof-of-concept with the hypothesis that measuring the release of secreted factors after cell transplantation, rather than the biodistribution of the cells alone, would be an alternative monitoring tool to understand the exposure of a subject to MSCs. By comparing cellular engraftment and the associated serum concentration of secreted factors released from the graft, we observed clear differences between the pharmacokinetics of MSCs and their secreted factors. Exploration of the effects of natural or engineered secreted proteins, active cellular secretion pathways, and clearance mechanisms revealed novel aspects that affect the systemic exposure of the host to secreted factors from a cellular therapeutic. We assert that a combined consideration of cell delivery strategies and molecular pharmacokinetics can provide a more predictive model for outcomes of MSC transplantation and potentially other transient cell therapeutics.
Conflict of interest statement
Figures


Similar articles
-
Transplanted interleukin-4--secreting mesenchymal stromal cells show extended survival and increased bone mineral density in the murine femur.Cytotherapy. 2018 Aug;20(8):1028-1036. doi: 10.1016/j.jcyt.2018.06.009. Epub 2018 Aug 2. Cytotherapy. 2018. PMID: 30077567 Free PMC article.
-
Bioactive factors secreted from mesenchymal stromal cells protect the intestines from experimental colitis in a three-dimensional culture.Cytotherapy. 2018 Dec;20(12):1459-1471. doi: 10.1016/j.jcyt.2018.06.007. Epub 2018 Oct 27. Cytotherapy. 2018. PMID: 30523788
-
Transplantation of human umbilical cord blood-derived mesenchymal stem cells or their conditioned medium prevents bone loss in ovariectomized nude mice.Tissue Eng Part A. 2013 Mar;19(5-6):685-96. doi: 10.1089/ten.TEA.2012.0047. Epub 2013 Jan 5. Tissue Eng Part A. 2013. PMID: 23215868 Free PMC article.
-
IFATS collection: in vivo therapeutic potential of human adipose tissue mesenchymal stem cells after transplantation into mice with liver injury.Stem Cells. 2008 Oct;26(10):2705-12. doi: 10.1634/stemcells.2008-0034. Epub 2008 Jun 5. Stem Cells. 2008. PMID: 18535155
-
The therapeutic potential of mesenchymal stem cells in lung cancer: benefits, risks and challenges.Cell Oncol (Dordr). 2019 Dec;42(6):727-738. doi: 10.1007/s13402-019-00459-7. Epub 2019 Jun 28. Cell Oncol (Dordr). 2019. PMID: 31254169 Review.
Cited by
-
Autologous, lentivirus-modified, T-rapa cell "micropharmacies" for lysosomal storage disorders.EMBO Mol Med. 2022 Apr 7;14(4):e14297. doi: 10.15252/emmm.202114297. Epub 2022 Mar 17. EMBO Mol Med. 2022. PMID: 35298086 Free PMC article.
-
Ectopic expression of BIRC5-targeting miR-101-3p overcomes bone marrow stroma-mediated drug resistance in multiple myeloma cells.BMC Cancer. 2019 Oct 21;19(1):975. doi: 10.1186/s12885-019-6151-x. BMC Cancer. 2019. PMID: 31638931 Free PMC article.
-
Extracellular matrix-based biomaterial scaffolds and the host response.Biomaterials. 2016 Apr;86:68-82. doi: 10.1016/j.biomaterials.2016.02.003. Epub 2016 Feb 3. Biomaterials. 2016. PMID: 26890039 Free PMC article. Review.
-
Enhanced In Vivo Delivery of Stem Cells using Microporous Annealed Particle Scaffolds.Small. 2019 Sep;15(39):e1903147. doi: 10.1002/smll.201903147. Epub 2019 Aug 13. Small. 2019. PMID: 31410986 Free PMC article.
-
Paracrine Proangiogenic Function of Human Bone Marrow-Derived Mesenchymal Stem Cells Is Not Affected by Chronic Kidney Disease.Stem Cells Int. 2019 Dec 23;2019:1232810. doi: 10.1155/2019/1232810. eCollection 2019. Stem Cells Int. 2019. PMID: 31933648 Free PMC article.
References
-
- Culme-Seymour EJ, Davie NL, Brindley DA, Edwards-Parton S, Mason C (2012) A decade of cell therapy clinical trials (2000–2010). Regen Med 7: 455–462. - PubMed
-
- Mason C, Brindley DA, Culme-Seymour EJ, Davie NL (2011) Cell therapy industry: billion dollar global business with unlimited potential. Regen Med 6: 265–272. - PubMed
-
- Brindley DA, Davie NL, Sahlman WA, Bonfiglio GA, Culme-Seymour EJ, et al. (2012) Promising growth and investment in the cell therapy industry during the first quarter of 2012. Cell Stem Cell 10: 492–496. - PubMed
-
- Meirelles LDS, Chagastelles PC, Nardi NB (2006) Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal Of Cell Science 119: 2204–2213. - PubMed
-
- Tang YL, Zhao Q, Zhang YC, Cheng LL, Liu MY, et al. (2004) Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regulatory Peptides 117: 3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

