Cross-presentation of synthetic long peptides by human dendritic cells: a process dependent on ERAD component p97/VCP but Not sec61 and/or Derlin-1
- PMID: 24587108
- PMCID: PMC3937416
- DOI: 10.1371/journal.pone.0089897
Cross-presentation of synthetic long peptides by human dendritic cells: a process dependent on ERAD component p97/VCP but Not sec61 and/or Derlin-1
Abstract
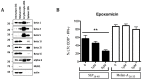
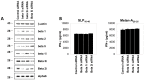
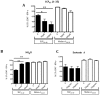
Antitumor vaccination using synthetic long peptides (SLP) is an additional therapeutic strategy currently under development. It aims to activate tumor-specific CD8(+) CTL by professional APCs such as DCs. DCs can activate T lymphocytes by MHC class I presentation of exogenous antigens - a process referred to as "cross-presentation". Until recently, the intracellular mechanisms involved in cross-presentation of soluble antigens have been unclear. Here, we characterize the cross-presentation pathway of SLP Melan-A16-40 containing the HLA-A2-restricted epitope26-35 (A27L) in human DCs. Using confocal microscopy and specific inhibitors, we show that SLP16-40 is rapidly taken up by DC and follows a classical TAP- and proteasome-dependent cross-presentation pathway. Our data support a role for the ER-associated degradation machinery (ERAD)-related protein p97/VCP in the transport of SLP16-40 from early endosomes to the cytoplasm but formally exclude both sec61 and Derlin-1 as possible retro-translocation channels for cross-presentation. In addition, we show that generation of the Melan-A26-35 peptide from the SLP16-40 was absolutely not influenced by the proteasome subunit composition in DC. Altogether, our findings propose a model for cross-presentation of SLP which tends to enlarge the repertoire of potential candidates for retro-translocation of exogenous antigens to the cytosol.
Conflict of interest statement
Figures







Similar articles
-
ISCOMATRIX adjuvant induces efficient cross-presentation of tumor antigen by dendritic cells via rapid cytosolic antigen delivery and processing via tripeptidyl peptidase II.J Immunol. 2009 Feb 1;182(3):1253-9. doi: 10.4049/jimmunol.182.3.1253. J Immunol. 2009. PMID: 19155470
-
Processing and cross-presentation of individual HLA-A, -B, or -C epitopes from NY-ESO-1 or an HLA-A epitope for Melan-A differ according to the mode of antigen delivery.Blood. 2010 Jul 15;116(2):218-25. doi: 10.1182/blood-2009-10-249458. Epub 2010 Apr 29. Blood. 2010. PMID: 20430956
-
Sec61 blockade by mycolactone inhibits antigen cross-presentation independently of endosome-to-cytosol export.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):E5910-E5919. doi: 10.1073/pnas.1705242114. Epub 2017 Jul 5. Proc Natl Acad Sci U S A. 2017. PMID: 28679634 Free PMC article.
-
Spotlight on TAP and its vital role in antigen presentation and cross-presentation.Mol Immunol. 2022 Feb;142:105-119. doi: 10.1016/j.molimm.2021.12.013. Epub 2021 Dec 29. Mol Immunol. 2022. PMID: 34973498 Free PMC article. Review.
-
Heat shock proteins in antigen trafficking--implications on antigen presentation to T cells.Int J Hyperthermia. 2009 Dec;25(8):617-25. doi: 10.3109/02656730902902183. Int J Hyperthermia. 2009. PMID: 19551545 Review.
Cited by
-
The functional role of L-fucose on dendritic cell function and polarization.Front Immunol. 2024 Apr 5;15:1353570. doi: 10.3389/fimmu.2024.1353570. eCollection 2024. Front Immunol. 2024. PMID: 38646527 Free PMC article.
-
Spatial separation of the processing and MHC class I loading compartments for cross-presentation of the tumor-associated antigen HER2/neu by human dendritic cells.Oncoimmunology. 2015 May 26;4(11):e1047585. doi: 10.1080/2162402X.2015.1047585. eCollection 2015 Nov. Oncoimmunology. 2015. PMID: 26985398 Free PMC article.
-
Cross-Presentation of Cell-Associated Antigens by MHC Class I in Dendritic Cell Subsets.Front Immunol. 2015 Jul 17;6:363. doi: 10.3389/fimmu.2015.00363. eCollection 2015. Front Immunol. 2015. PMID: 26236315 Free PMC article. Review.
-
Distinct Subcellular Compartments of Dendritic Cells Used for Cross-Presentation.Int J Mol Sci. 2019 Nov 9;20(22):5606. doi: 10.3390/ijms20225606. Int J Mol Sci. 2019. PMID: 31717517 Free PMC article. Review.
-
Virus-Induced CD8+ T-Cell Immunity and Its Exploitation to Contain the SARS-CoV-2 Pandemic.Vaccines (Basel). 2021 Aug 18;9(8):922. doi: 10.3390/vaccines9080922. Vaccines (Basel). 2021. PMID: 34452047 Free PMC article.
References
-
- Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A (1994) Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 12: 337–365. - PubMed
-
- Melief CJ (2008) Cancer immunotherapy by dendritic cells. Immunity 29: 372–383. - PubMed
-
- Steinman RM, Banchereau J (2007) Taking dendritic cells into medicine. Nature 449: 419–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

