Abrogation of TNFα production during cancer immunotherapy is crucial for suppressing side effects due to the systemic expression of IL-12
- PMID: 24587231
- PMCID: PMC3938584
- DOI: 10.1371/journal.pone.0090116
Abrogation of TNFα production during cancer immunotherapy is crucial for suppressing side effects due to the systemic expression of IL-12
Abstract
For more than a decade, the cytokine interleukin-12 (IL-12) has been utilized, either alone or in combination with other drugs, as a treatment for cancer. The numerous anti-tumor properties of IL-12 still generate interest in the clinical use of this cytokine, even though it has demonstrated toxicity when administrated systemically. As an approach to overcome this toxicity, numerous laboratories have attempted to induce IL-12 expression at the site of the tumor. However for tumors that are difficult to remove surgically or for the treatment of disseminated metastases, systemic expression of this cytokine still remains as the most efficient method of administration. Nevertheless, finding alternative approaches for the use of IL-12 in the treatment of cancer and unraveling the basis of IL-12-side effects remain a challenge. In the present work we demonstrate that systemic expression of IL-12 through hydrodynamic injection of IL-12 cDNA is able to induce different types of liver lesions associated with a toxic pathology. However we report here that hepatic toxicity is diminished and survival of mice enhanced in the absence of tumor necrosis factor alpha (TNFα). This observation is in contrast to several murine models and clinical trials that postulate interferon gamma (IFNγ) as the main cytokine responsible for IL-12 toxicity. Moreover, our work demonstrates that when IL-12 cDNA is co-injected with IL-18 cDNA or when mice are pre-treated with a low dose of IL-12 cDNA prior to receiving a high dose of IL-12 cDNA, systemic levels of TNFα are almost completely abrogated, resulting in improved survival and less hepatic damage. Importantly, abrogation of TNFα signaling does not affect the strong anti-tumor activity of IL-12. Thus, neutralizing TNFα with antagonists already approved for human use offers a promising approach to abrogate IL-12 side effects during the use of this cytokine for the treatment of cancer.
Conflict of interest statement
Figures
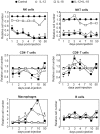


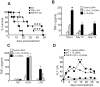



References
-
- Rook AH, Zaki MH, Wysocka M, Wood GS, Duvic M, et al. (2001) The role for interleukin-12 therapy of cutaneous T cell lymphoma. Ann N Y Acad Sci 941: : 177–184. PubMed: 11594571. - PubMed
-
- Hamid O, Solomon JC, Scotland R, Garcia M, Sian S, et al. (2007) Alum with interleukin-12 augments immunity to a melanoma peptide vaccine: correlation with time to relapse in patients with resected high-risk disease. Clin Cancer Res 13: : 215–222. PubMed: 17200357. - PubMed
-
- Lenzi R, Edwards R, June C, Seiden MV, Garcia ME, et al. (2007) Phase II study of intraperitoneal recombinant interleukin-12 (rhIL-12) in patients with peritoneal carcinomatosis (residual disease < 1 cm) associated with ovarian cancer or primary peritoneal carcinoma.J Transl Med 5: : 66. doi: 10.1186/1479-5876-5-66. PM:18076766. - PMC - PubMed
-
- Yarchoan R, Pluda JM, Wyvill KM, Aleman K, Rodriguez-Chavez IR, et al. (2007) Treatment of AIDS-related Kaposi's sarcoma with interleukin-12: rationale and preliminary evidence of clinical activity. Crit Rev Immunol 27: : 401–414. PubMed: 18197804. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

